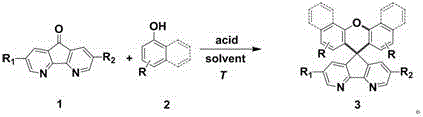
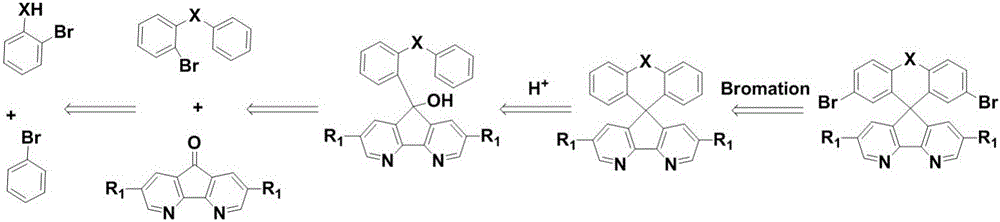
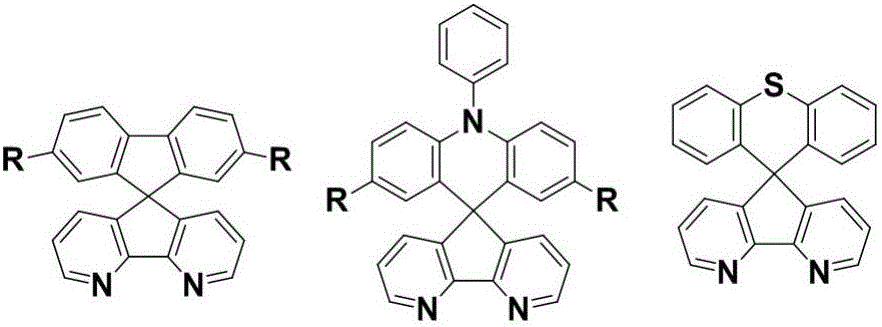
Synthesis method of azafluorene spiro aromatic hydrocarbon
A technology of spiroaromatics and synthesis methods, which is applied in the field of efficient preparation of preparation methods, can solve the problems of long synthetic routes, inability to directly prepare azafluorene spiroaromatics with flexible substituents, limited expansion of substrates, etc., and achieve low cost , easy industrial production, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0039] Example 1: Preparation of 3a with azafluorenone and 1-naphthol as raw materials
[0040]
[0041] First add azafluorenone 1a (0.18g, 1mmol), dichlorobenzene 2ml and methanesulfonic acid (0.26ml, 4mmol) to the reaction flask in sequence, keep stirring at 35°C for about 30min, then add 1-naphthol (0.36g, 2.5mmol), the reaction temperature was raised to 150°C for a period of time, and the raw materials were basically reflected by thin-layer chromatography monitoring. Cool and stand, add saturated potassium carbonate solution to the reaction flask to quench the reaction. Pour the mixed solution into potassium carbonate solution or deionized water, extract with dichloromethane, collect the organic phase (dichloromethane extract), dry with anhydrous sodium sulfate, remove the desiccant by suction filtration, and distill off the solvent under reduced pressure, the crude product Azafluorene spiroarene 3a (0.21 g, 50%) with high purity can be obtained by silica gel column ch...
example 2
[0042] Example 2: Preparation of 3b with azafluorenone and p-bromophenol as raw materials
[0043]
[0044]First add azafluorenone 1a (0.18g, 1mmol), dichlorobenzene 2ml and methanesulfonic acid (0.26ml, 4mmol) to the reaction flask in sequence, keep stirring at 35°C for about 30min, then add p-bromophenol ( 0.43g, 2.5mmol), the reaction temperature was increased to 150°C for a period of time, and the raw materials were basically reflected by thin-layer chromatography monitoring. Cool and stand, add saturated potassium carbonate solution to the reaction flask to quench the reaction. Pour the mixed solution into potassium carbonate solution or deionized water, extract with dichloromethane, collect the organic phase (dichloromethane extract), dry with anhydrous sodium sulfate, remove the desiccant by suction filtration, and distill off the solvent under reduced pressure, the crude product Azafluorene spiroarene 3b (0.20 g, 41%) with high purity can be obtained by silica gel ...
example 3
[0045] Example 3: Preparation of 3c with azafluorenone and p-bromophenol as raw materials
[0046]
[0047] First add azafluorenone 1a (0.18g, 1mmol), dichlorobenzene 2ml and methanesulfonic acid (0.26ml, 4mmol) to the reaction flask in sequence, keep stirring at 35°C for about 30min, then add p-bromophenol ( 0.43g, 2.5mmol), the reaction temperature was increased to 150°C for a period of time, and the raw materials were basically reflected by thin-layer chromatography monitoring. Cool and stand, add saturated potassium carbonate solution to the reaction flask to quench the reaction. Pour the mixed solution into potassium carbonate solution or deionized water, extract with dichloromethane, collect the organic phase (dichloromethane extract), dry with anhydrous sodium sulfate, remove the desiccant by suction filtration, and distill off the solvent under reduced pressure, the crude product Azafluorene spiroarene 3c (0.17g, 43%) with high purity can be obtained by silica gel ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com