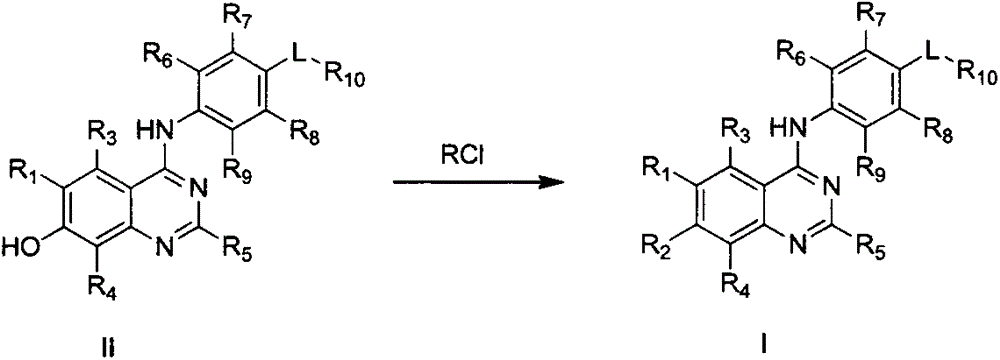
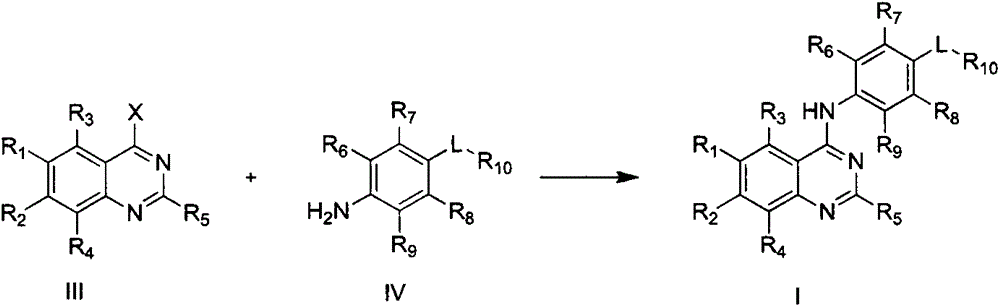
4-substituted anilinoquinazoline derivatives, and preparation method and application thereof
A technology of aniline quinazolines and aniline quinazolines, applied in the field of drug synthesis, can solve problems such as easy drug resistance, and achieve strong anti-tumor cell proliferation activity and strong inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 N-(4-nitrophenyl)benzamide
[0030] In an ice bath, under the protection of nitrogen, 4-nitroaniline (2.0mmol, 276mg), potassium carbonate (2.4mmol, 331mg) and anhydrous THF 10ml were added to a 50ml two-necked flask, and then benzoyl chloride (0.28ml , 2.4mmol), after the dropwise addition was completed, the reaction was carried out at room temperature for 2 hours. Add 10% dilute hydrochloric acid (10ml) to the reaction solution, stir, and a large amount of yellow solid precipitates out, let stand, filter with suction, add water to wash the filter cake, and dry to obtain 407 mg of yellow solid, which is N-(4-nitrophenyl)benzene Formamide, yield 84%.
[0031] Replace benzoyl chloride with the corresponding substituted benzoyl chloride, and use the same method to prepare: N-(4-nitrophenyl)-2-chlorobenzamide, yellow solid, yield 97.6%; N-(4-nitrophenyl) N-(4-nitrophenyl)-3-chlorobenzamide, yellow solid, yield 98%; N-(4-nitrophenyl)-4-chlorobenzamide, yellow so...
Embodiment 2
[0032] Example 2 N-(4-aminophenyl)benzamide
[0033] Add a mixed solvent of N-(4-nitrophenyl)benzamide (1.5mmol, 363mg), zinc powder (7.5mmol, 488mg) and 11ml of methanol / water (V / V=10 / 1) into a 50ml reaction flask In, and then slowly dropwise added glacial acetic acid (15mmol, 0.9ml), after the dropwise addition was completed, heated to reflux for 1 hour. After the reaction is complete, filter with suction, add a small amount of methanol to wash, and then concentrate the filtrate. The crude product was recrystallized from methanol water and dried to obtain 261 mg of a yellow solid, namely N-(4-aminophenyl)benzamide, with a yield of 72%.
[0034] With N-(4-nitrophenyl)-2-chlorobenzamide instead of N-(4-nitrophenyl)benzamide, the same method was used to prepare N-(4-aminophenyl)-2- Chlorobenzamide, yellow solid, yield 80%; replace N-(4-nitrophenyl)benzamide with N-(4-nitrophenyl)-3-chlorobenzamide, and use the same method Preparation of N-(4-aminophenyl)-3-chlorobenzamide, y...
Embodiment 3
[0035] Example 3 N-(4-nitrophenyl)phenylurea
[0036] Dissolve triphosgene (2.97g, 10mmol) in 20ml of dichloromethane, add dropwise a solution of 4-nitroaniline (1.38g, 10mmol) and triethylamine (2.8ml) in dichloromethane (10ml), and dropwise, Continue to stir for 30 minutes, then add aniline (0.93g, 10mmol), heat up to 40°C, stir and reflux for 30 minutes, spin dry dichloromethane, add acetone: water (2:1) 30ml, shake fully, there is solid precipitation, suction filtration, The filter cake was washed twice with 5 ml of acetone:water (1:1), and dried to obtain 1.58 g of a yellow solid, namely N-(4-nitrophenyl)phenylurea, with a yield of 61.7%.
[0037] Replace aniline with 4-chloroaniline, and use the same method to prepare N-(4-nitrophenyl)-4-chlorophenylurea, a yellow solid, with a yield of 58.6%; use 3-chloroaniline instead of aniline, and use the same method Preparation of N-(4-nitrophenyl)-3-chlorophenylurea, yellow solid, yield 62.1%; replace aniline with 2-methylanilin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com