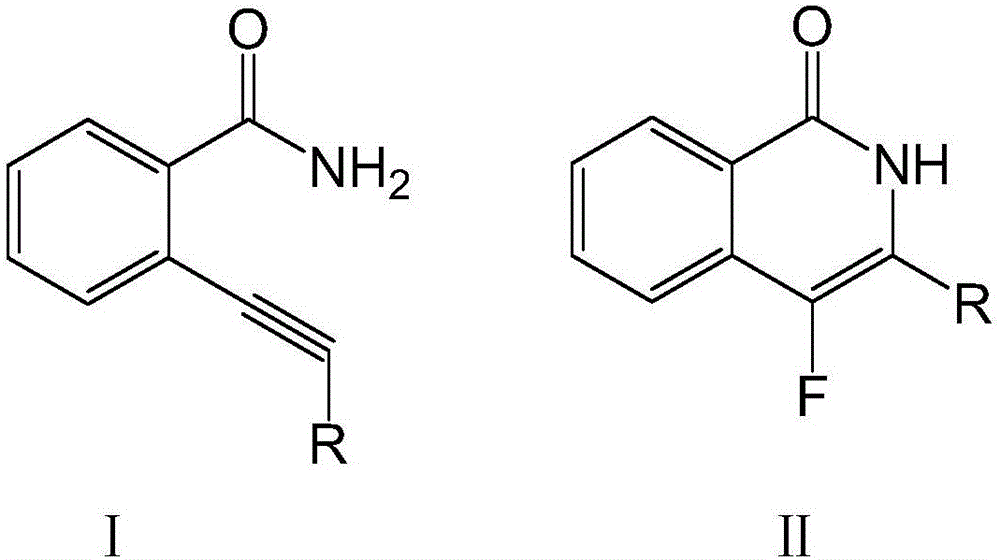
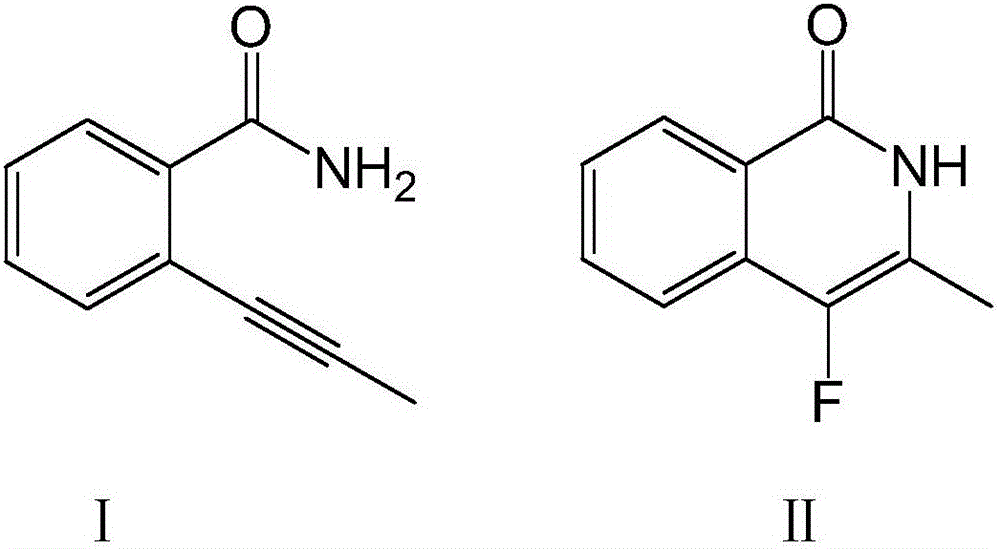
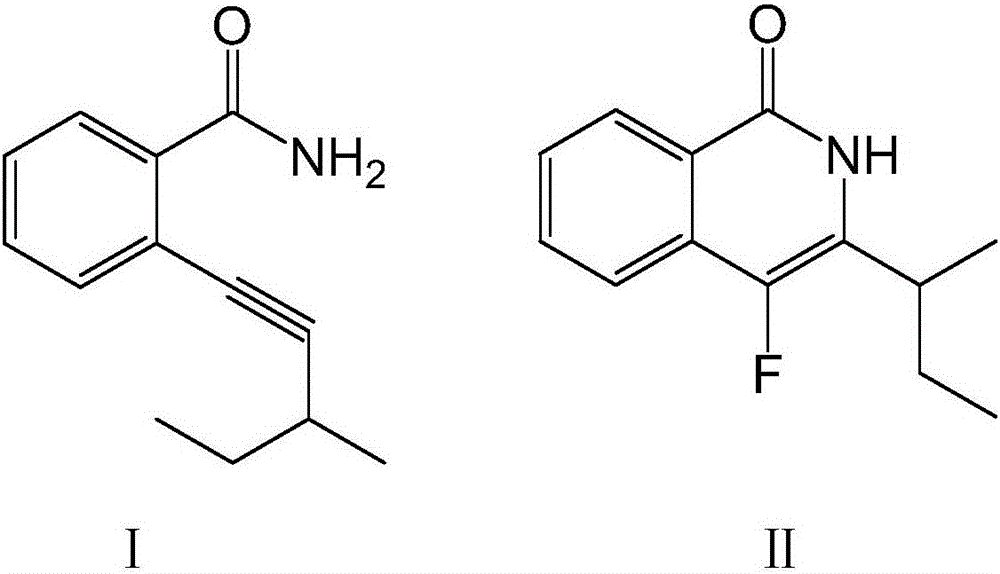
Fluoroisoquinolone compound and synthesis method thereof
A technology of isoquinolinone and synthesis method, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalyst, chemical/physical process, etc., can solve the requirements of high reaction conditions, harsh environmental conditions, The problem of high production cost, to achieve the effect of broad industrial prospects, high production efficiency and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] Dissolve 100 mmol of compound I in the above formula in ether, add 150 mmol of fluorinating agent and 7 mmol of catalyst, raise the temperature to 40°C, stir at a speed of 70 rpm, and continue the reaction for 8 hours. After washing with water for 3 times, adding n-hexane for extraction, drying and concentrating, passing through a silica gel chromatography column, and washing with an equal volume ratio of acetone-n-hexane mixture, the compound II in the above formula was obtained.
[0027] The purity of the target product is 89.3%, and the yield is 93.7%.
Embodiment 2
[0029]
[0030] Dissolve 100 mmol of compound I in the above formula in ether, add 160 mmol of fluorinating agent and 8 mmol of catalyst, raise the temperature to 50°C, stir at a speed of 60 rpm, and continue the reaction for 8 hours. After washing with water for 3 times, adding n-hexane for extraction, drying and concentrating, passing through a silica gel chromatography column, and washing with an equal volume ratio of acetone-n-hexane mixture, the compound II in the above formula was obtained.
[0031] The purity of the target product is 89.8%, and the yield is 94.2%.
Embodiment 3
[0033]
[0034] Dissolve 100 mmol of compound I in the above formula in ether, add 170 mmol of fluorinating agent and 9 mmol of catalyst, raise the temperature to 60°C, stir at a speed of 50 rpm, and continue the reaction for 8 hours. After washing with water for 3 times, adding n-hexane for extraction, drying and concentrating, passing through a silica gel chromatography column, and washing with an equal volume ratio of acetone-n-hexane mixture, the compound II in the above formula was obtained.
[0035] Wherein the purity of the target product is 92.1%, and the yield is 95.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com