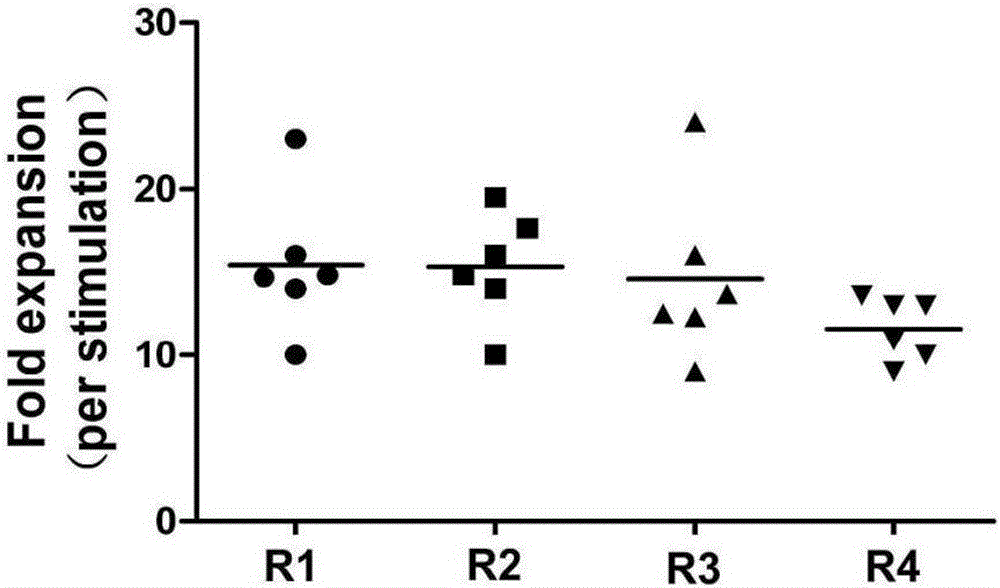
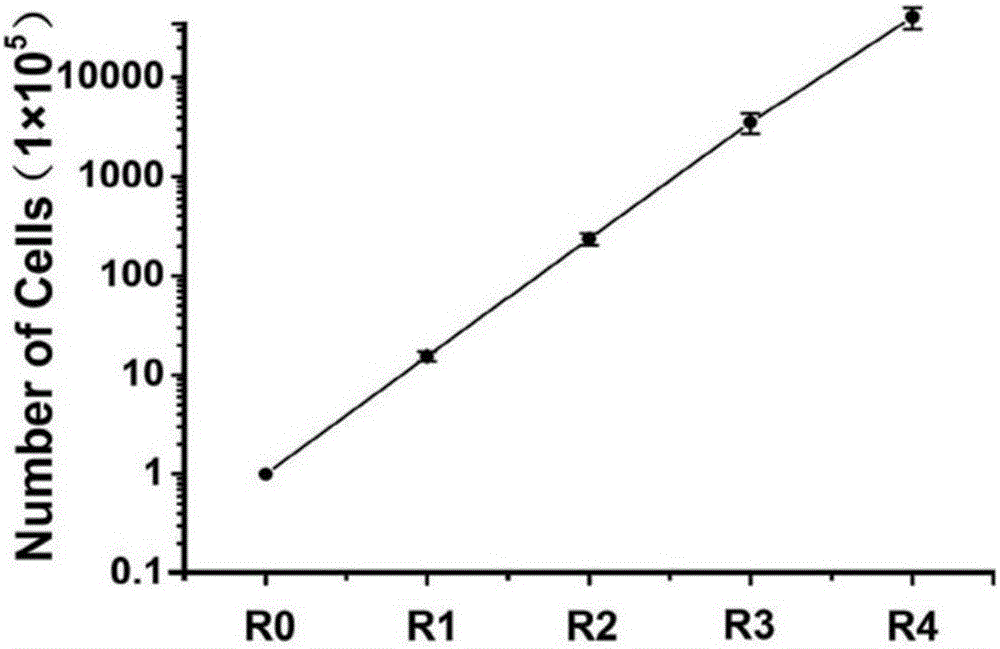
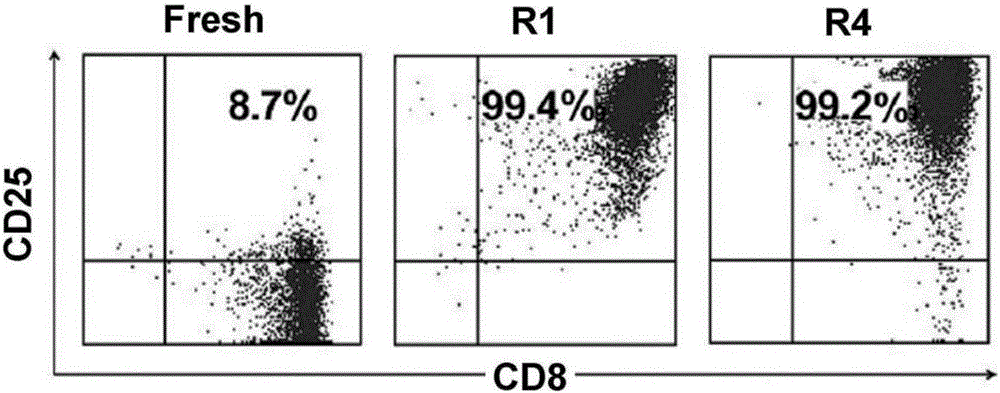
Method for inducing in-vitro expansion of CD8<+> regulatory T cells by immunosuppressants
An in vitro amplification and immunosuppression technology, applied in the field of immunology, can solve the problems of difficult clinical application, long amplification time, difficulty in meeting GMP requirements, etc., and achieve the effect of great clinical application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Material:
[0034] Healthy human mononuclear cells (PBMC); AB blood type human serum; lymphocyte separation medium (Ficoll); 1640 culture medium; phosphate buffer saline (PBS); fetal bovine serum (FBS); human CD8 + Cell sorting kit; Anti-CD3 / CD28 monoclonal antibody coated immunostimulatory magnetic beads; Recombinant human interleukin 2 (IL-2); Transforming growth factor 1 (TGF-β1); Rapamycin (RAPA ).
[0035] method:
[0036] 1. Human CD8 + Isolation and purification of T cells (sterile environment)
[0037] 1.1 Separation of PBMC cells from buffy coat blood by Ficoll density gradient centrifugation
[0038] Centrifuge a unit of buffy coat blood at 3500rpm for 15 minutes; absorb the concentrated buffy coat after centrifugation, dilute it with the original plasma, mix it and slowly resuspend it on Ficoll, and suck out the buffy coat after density gradient centrifugation; add PBS to wash to obtain PBMC cells .
[0039] 1.2 Using human CD8 + T cell sorting kit for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com