Sacubitril sodium crystal forms, preparation method and application thereof
A technology of sacubitril sodium and sacubitril, which is applied in the preparation of organic compounds, the preparation of carboxylic acid amides, chemical instruments and methods, etc., can solve problems such as unfavorable medication adjustment, fixed composition ratio and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] Example 1: Preparation of Sacubitrone Sodium Form A
[0215] Dissolve 0.09g (2.19mmol) of sodium hydroxide in 2ml of absolute ethanol at 40-45°C; dissolve 1.00g (2.43mmol) of sacubiqu in 10ml of absolute ethanol at room temperature. Under stirring, the ethanol solution of the above-mentioned sodium hydroxide was added dropwise to the absolute ethanol solution of Sacubitril to obtain a clear solution. At 55-60°C, add methyl tert-butyl ether dropwise until solid precipitates, then cool. Filtrate, wash the filter cake with methyl tert-butyl ether, and dry under reduced pressure at 105-110°C to obtain crystalline form A of sacubitronic sodium.
[0216] Moisture content: 0.64%.
[0217] 1 H NMR (400MHz, CD 3 OD)δ:1.143-1.161(d,3H),1.208-1.242(t,3H),1.453-1.525(m,1H),1.886-1.966(m,1H),2.394-2.441(m,4H),2.554 -2.608(m,1H),2.758-2.801(m,2H),4.042-4.156(m,3H),7.286-7.335(m,3H),7.403-7.441(m,2H),7.538-7.818(m, 4H).
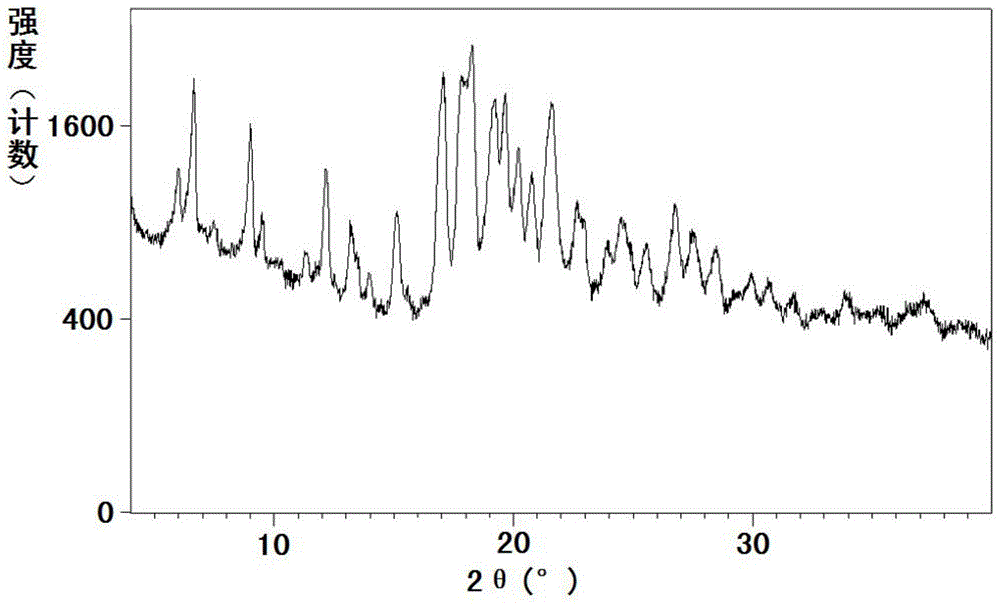
[0218] The measured powder X-ray diffraction pattern is ...
Embodiment 2
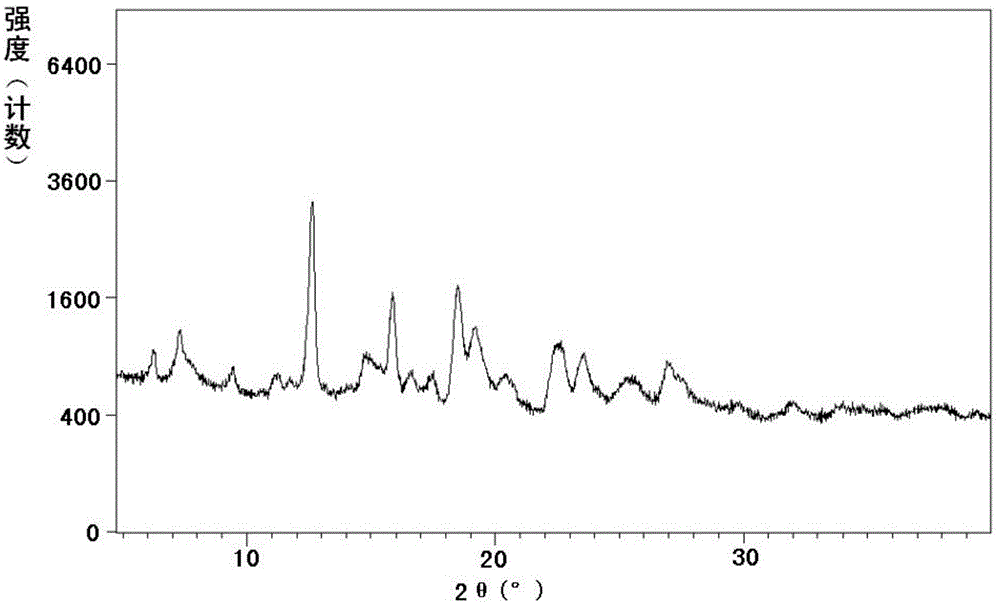
[0221] Example 2: Preparation of Sacubitrone Sodium Form A
[0222] Dissolve 0.09g (2.19mmol) of sodium hydroxide in 2ml of absolute ethanol at 40-45°C; dissolve 1.00g (2.43mmol) of sacubiqu in 10ml of absolute ethanol at room temperature. Under stirring, the ethanol solution of the above-mentioned sodium hydroxide was added dropwise to the absolute ethanol solution of Sacubitril to obtain a clear solution. At 55-60°C, add methyl tert-butyl ether dropwise until solid precipitates, then cool. After filtering, the filter cake was washed with methyl tert-butyl ether, and dried under reduced pressure at 20-30°C to obtain crystalline form A of sacubitronic sodium.
[0223] Moisture content: 3.2%.
Embodiment 3
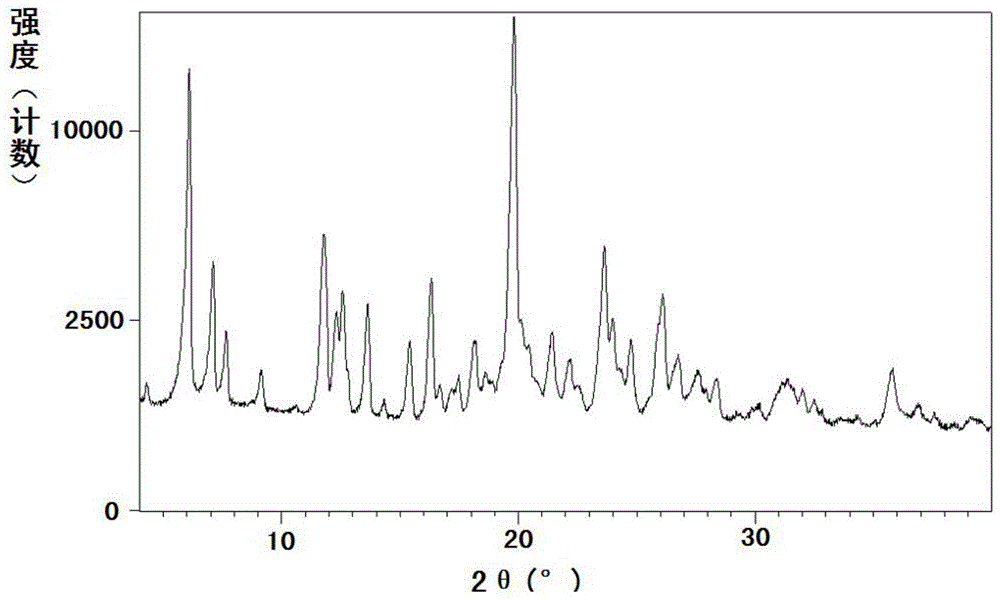
[0224] Example 3: Preparation of Sacubitrone Sodium Form B
[0225] Dissolve 0.09g (2.19mmol) of sodium hydroxide in 2ml of absolute ethanol at 40-45°C; dissolve 1.00g (2.43mmol) of sacubiqu in 10ml of absolute ethanol at room temperature. Under stirring, the ethanol solution of the above-mentioned sodium hydroxide was added dropwise to the absolute ethanol solution of Sacubitril to obtain a clear solution. Concentrate under reduced pressure at 40-45°C to an oil. Dissolve the concentrated oil in 10ml of ethyl acetate at 55-60°C, then add 5ml of petroleum ether (60-90°C) dropwise, and cool. After filtering, the filter cake was washed with petroleum ether (60-90°C), and dried under reduced pressure at 105-110°C to obtain crystalline form B of sacubitronic sodium.
[0226] The moisture content is 0.39%.
[0227] 1 H NMR (400MHz, CD 3 OD)δ:1.144-1.181(d,3H),1.206-1.241(t,3H),1.464-1.526(m,1H),1.886-1.966(m,1H),2.395-2.442(m,4H),2.554 -2.615(m,1H),2.758-2.801(m,2H),4.059-4.15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com