Diagnostic reagents for improved in vivo or in vitro cell-mediated immunological diagnosis of tuberculosis
A technology for tuberculosis and reagent kits, applied in the field of immunodiagnostic compositions for tuberculosis, which can solve the problems of difficulty and difficulty in increasing the sensitivity of non-specific responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Example 1: Preliminary Selection of Antigens
[0129] The T-cell antigens chosen for immunodiagnosis of TB should be specific for M. tuberculosis infection to avoid interference from BCG vaccination and most prevalent atypical mycobacteria. At the same time, avoiding ESAT-6 is important given that it is present in many novel vaccines against TB. As described above in the Background of the Invention, through an extensive and rigorous down-selection approach based on theoretical considerations, practical testing and literature searches of hundreds of selected potential antigens, we have no more than 9 Mycobacterium tuberculosis antigens were used for further testing. They are:
[0130] CFP10(Rv3874). The 10-kDa culture filtrate antigen together with ESAT-6 is the basis of the current cell-based diagnostic blood test for M. tuberculosis infection by the IFN-γ release assay (IGRA). CFP10 is an immunodominant Mycobacterium tuberculosis antigen, and the diagnostic specific...
Embodiment 2
[0140] Example 2. Selection of antigens
[0141] Recognition of the seven antigens listed above in TB patients or latently infected individuals was tested in two separate studies in Egypt and Greenland. In both studies ESAT-6 (Rv3875) was also included as a comparator and reference antigen. In addition, PPD was included in Egypt as an example of non-specific antigenic stimulation.
[0142] Freshly sampled diluted whole blood was restimulated with peptides selected from the listed antigens, and responses to the peptide pool of ESAT-6 and CFP10 were included as benchmarks.
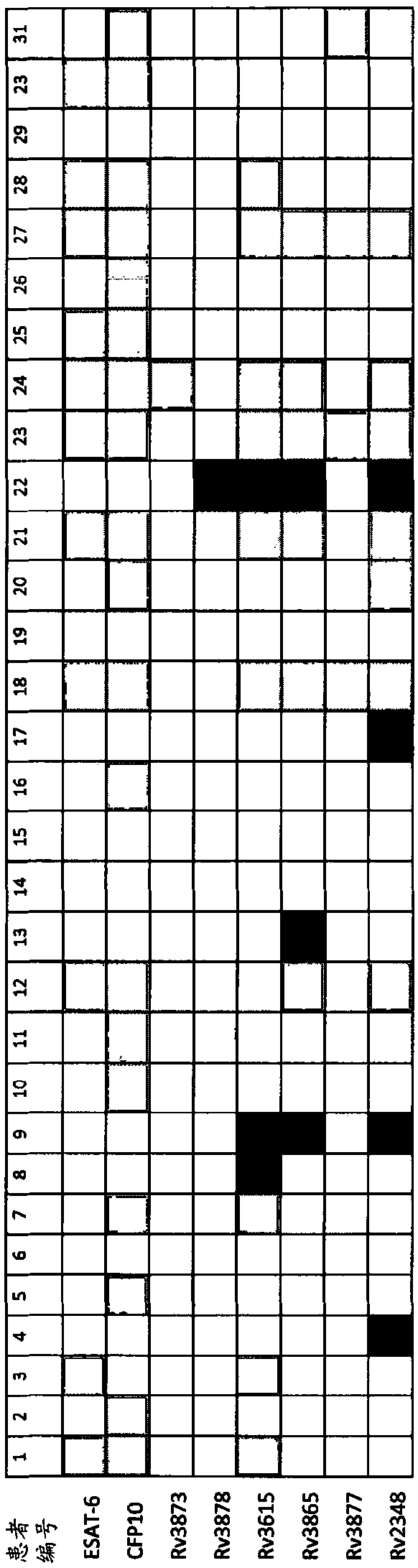
[0143] In the Egyptian study, 34 volunteer donors (8 female and 26 male) were included as positive controls. 32 of these were diagnosed with TB disease with documented positive sputum cultures (subjects 3-34). Two had latent TB (subjects 1 and 2). In addition, 30 endemic negative control donors (5 female and 26 male) were included. They were all presumptively BCG vaccinated, had no history of TB disease...
Embodiment 3
[0146] Example 3. CFP10 and 3615c are comparable to CFP10 and ESAT-6
[0147] The diagnostic performance of combining CFP10 and Rv3615c was then compared with that of combining CFP10 and ESAT-6. We included whole blood samples from 35 individuals from Greenland, 18 of the 35 individuals had latent M. tuberculosis infection, defined as a positive Quantiferon test and / or demonstrated exposure to M. tuberculosis and tuberculosis The plain skin test was converted, and 17 patients were microbiologically confirmed as TB. A single aliquot of 200 μl undiluted whole blood was treated with overlapping peptides representing CFP10 (SEQID1) or Rv3615 (SEQID15-18) or ESAT-6 (SEQID51) at a final concentration of 5 μg / ml in a humidified 37°C incubator Stimulate for 7 days. Negative control samples (nil) were prepared in parallel. After incubation, plasma supernatants were isolated and ELISA was used to determine the levels of IFN-γ.
[0148] By summing the measured levels of antigen-speci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com