Xylanase thermosensitive mutant and preparing method and application thereof
A xylanase mutation, xylanase technology, applied in the field of xylanase heat-sensitive mutants and preparation thereof, can solve the problems of easy thermal inactivation, poor thermal stability of the enzyme, etc., and achieve the effect of low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Construction of mutant library
[0032] Genomes of Arthrobacter sp. and Lechevalieria sp. were extracted according to the instructions of the GENESTAR Bacterial Genome Extraction Kit. According to the GenBank xylanase sequences JQ863105 and JF745868, primers 5'GTCTCGGCCCCGCCGGACGT3' and 5'GGCTCGCTTCGCCAGCGTGG3' were designed, and PCR amplification was performed using the Lechevalieria sp. genome as a template to obtain the xylanase gene xynAHJ3, In addition, primers 5'GTGCAGCCGGAGGAAAAACG3' and 5'GATGAAGGCAGGATCCGGGGT3' were designed, and the Arthrobacter sp. genome was used as a template for PCR amplification to obtain the xynAGN16L xylanase gene.
[0033] The PCR reaction parameters were: denaturation at 94°C for 5 minutes; then denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec, extension at 72°C for 1 min and 30 sec, and after 30 cycles, incubation at 72°C for 10 min.
[0034] Using the above PCR product as a template, the error-prone PCR kit ...
Embodiment 2
[0037] Example 2: Screening of Mutants
[0038]Take 2 μL of bacterial liquid from the 96-well cell culture plate where the mutant library is stored, and inoculate it in 200 μL / well liquid LB culture medium (containing 100 μgmL -1 Amp) in a 96-deep-well plate at 37°C with shaking at 200rpm until OD 600 >1.0 (about 20h), add 2mMIPTG and 100μgmL -1 Amp was induced overnight at 20° C. with 160 rpm in 200 μL liquid LB culture solution. Add 40 μL / well of PopCulture after induction TM Cell lysate, shake and lyse cells at 25°C for 30 minutes. Take 50 μL of McIlvaine buffer (pH=7.0) containing 1.0% (w / v) beech xylan and 50 μL of cell lysate, and react in a 96-deep well plate in a 70° C. incubator for 2 h. After the reaction is over, add 150 μL of DNS reagent to terminate the reaction, incubate in a 140°C incubator for more than 20 minutes and cool to room temperature, and use a microplate reader to read the OD 540nm The value of E.coliBL21-Gold (DE3) strain lysate reaction group c...
Embodiment 3
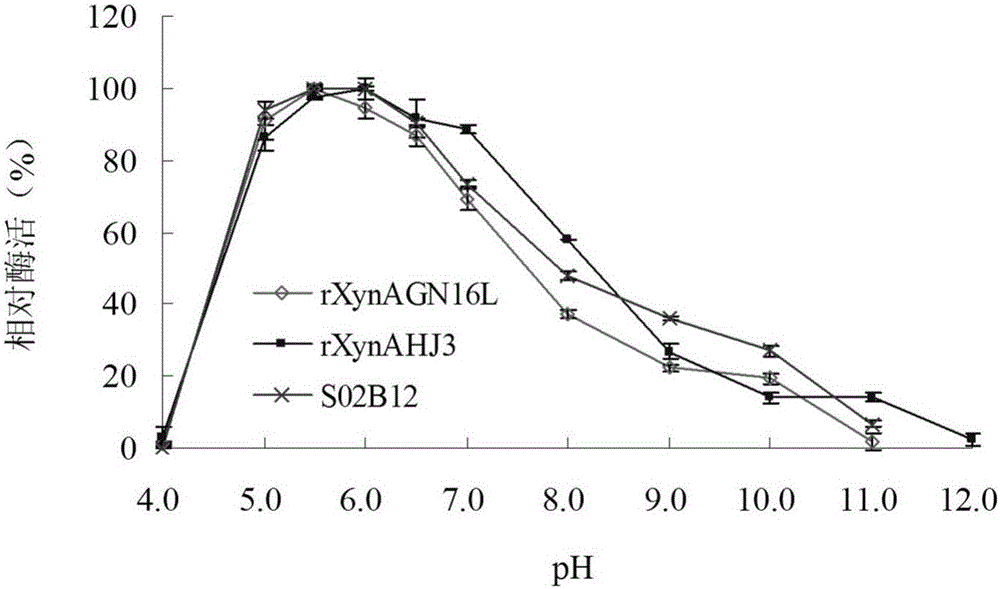
[0039] Embodiment 3: Enzyme preparation of mutant S02B12 and wild enzyme rXynAGN16L and rXynAHJ3
[0040] Mutant S02B12, wild enzymes rXynAGN16L and rXynAHJ3 were inoculated in LB (containing 100 μg mL -1 Amp) medium, shake rapidly at 37°C for 16h. Then this activated bacterial solution was inoculated into fresh LB (containing 100 μg mL -1 Amp) culture medium, rapid shaking culture for about 2–3h (OD 600 After reaching 0.6–1.0), add IPTG at a final concentration of 0.1 mM for induction, and continue shaking culture at 20° C. for about 20 h. Centrifuge at 12000rpm for 5min to collect the bacteria. After suspending the cells with an appropriate amount of pH=7.0 Tris-HCl buffer solution, the cells were ultrasonically disrupted in a low-temperature water bath. After the crude enzyme solution concentrated in the cells was centrifuged at 13000rpm for 10min, the supernatant was aspirated and the target protein was affinity-purified with Nickel-NTAAgarose and 0-500mM imidazole res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com