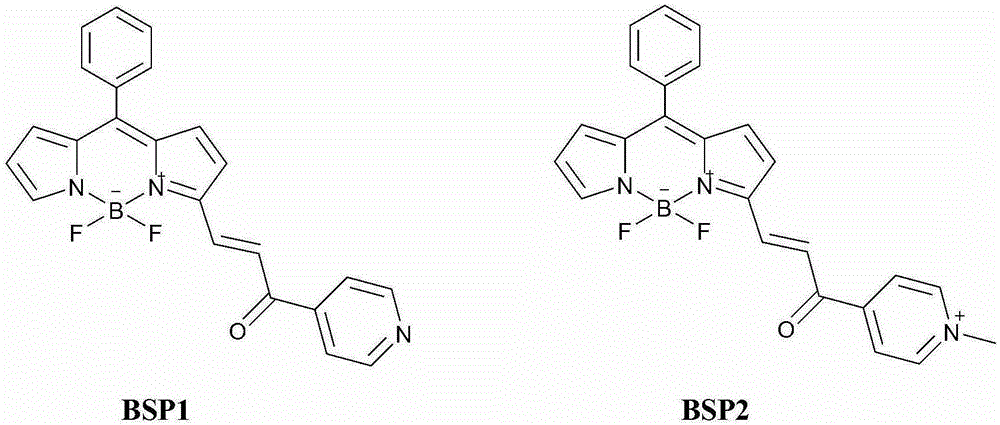Boron dipyrromethene compound containing alpha,beta-unsaturated ketone, and application of same in detection of sulfite
The technology of ketofluoroboron pyrrole and fluoroboron pyrrole is applied to α, β-unsaturated ketofluoroboron pyrrole and its application in sulfite detection, and can solve the problem of reduced measurement accuracy, measurement signal fluctuation, etc. Problems such as low detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
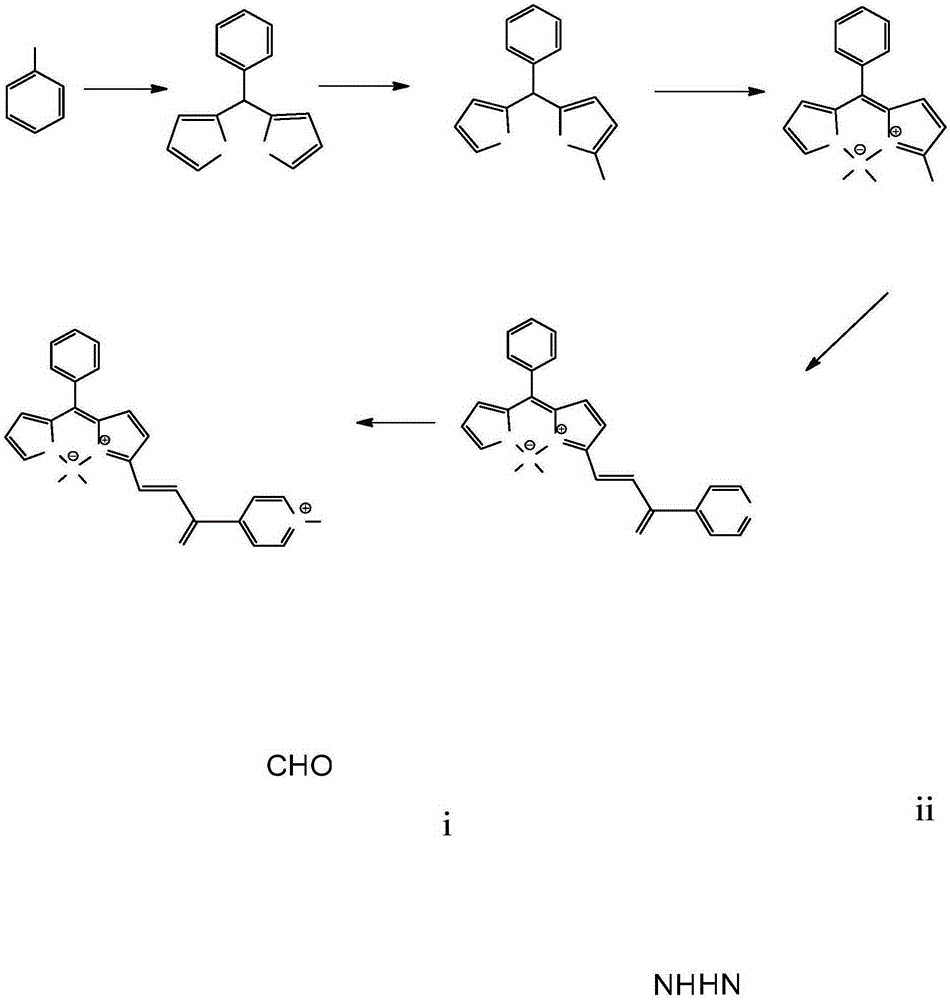
[0042] 1. Synthesis of compounds BSP1 and BSP2:
[0043] (1) Synthesis of Compound 1: Weigh 1.0g of benzaldehyde (9.43mmol) and 1.9g of pyrrole (28.29mmol) in a 250mL single-necked flask, add 150mL of ultrapure water, and add 7 to 8 drops of hydrochloric acid dropwise under stirring, The solution quickly turned milky white, and the above reaction solution was stirred in an ice bath for 4 hours, followed by TLC until the raw materials disappeared, and a large amount of solids were precipitated in the solution, filtered, and the solids were washed with ultrapure water and petroleum ether to obtain gray-green solids, the product with anhydrous Na 2 SO 4 Drying; the crude product was separated through a silica gel column (petroleum ether: dichloromethane = 1:1), and the solvent was removed by rotary evaporation to obtain 1.79 g of a light yellow solid. 1 HNMR (400MHz, CDCl 3 )δ(ppm): 9.51(s, 2H), 8.93~8.80(m, 6H), 8.28(s, 2H), 7.75(d, J=2.69Hz, 2H), 7.51(s, 2H).
[0044] (2) S...
Embodiment 2
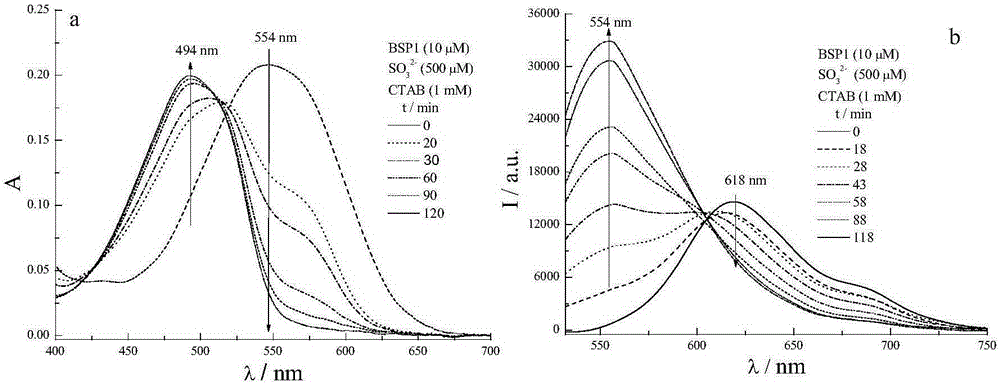
[0053] Determination of sulfite in buffer solution-CTAB system:
[0054] Accurately weigh a certain amount of compound BSP1 and dissolve it in 10mL N,N-dimethylformamide to prepare a 30mM stock solution and store it at low temperature; accurately weigh a certain amount of SO 3 2- Dissolve in 10mL phosphate buffer solution (PBS, 20mM, pH=7.4) to prepare 30mM sulfite stock solution; accurately weigh a certain mass of CTAB and dissolve in 10mL buffer solution to prepare 1mM CTAB-PBS stock solution . Accurately pipette 33.3 μL of the stock solution of compound BSP1 into a 10 mL volumetric flask, dilute to the mark with the CTAB-PBS stock solution, and obtain a buffer solution of 10 μL of BSP1. Add 167 μL of sulfite stock solution to the above solution, measure the fluorescence and ultraviolet spectra of the probe solution at different times, and obtain the time response curve of probe BSP1 to sulfite in the CTAB buffer solution system after data processing. Add different volume...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com