Quality control method and apparatus for electronic medical record
A quality control method and technology of electronic medical records, applied in the field of medical system informatization, can solve the problems of low efficiency of quality and air flow, manual review, inability to timely reflect the quality of medical records, etc., and achieve the effect of improving efficiency and simplifying the processing process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
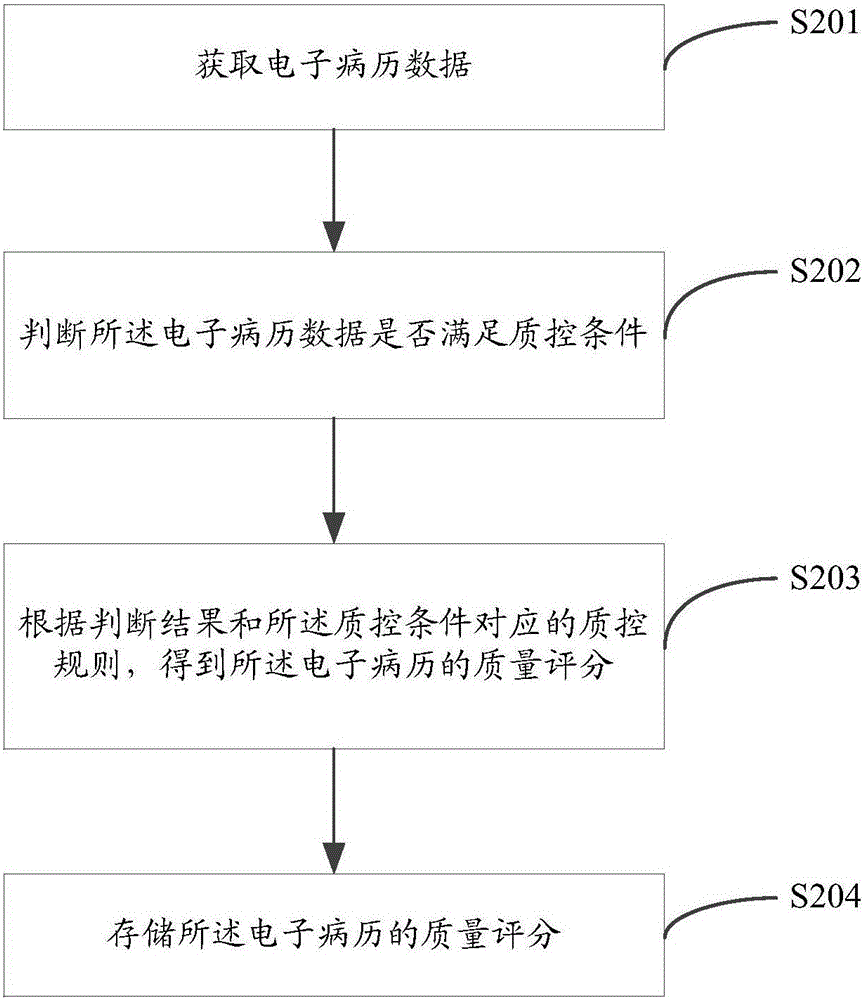
[0036] figure 2 The flow chart of a quality control method for electronic medical records provided by an embodiment of the present invention is shown, and the details are as follows:
[0037] In step S201, electronic medical record data is acquired.
[0038] Wherein, the electronic medical record data is structured electronic medical record data. The electronic medical record data is a collection of patient information in a structured electronic medical record with a four-layer storage structure. The four-layer storage structure includes metadata, data groups, document segments and medical record files. The specific actual application conditions are as follows :
[0039] 1) Metadata: including symptom metadata, anatomical part metadata, time metadata, quantity metadata, logical judgment metadata, etc. For example, "diarrhea" belongs to symptom metadata.
[0040] 2) Data group: composed of several metadata, for example, a paragraph describing the symptom of "diarrhea" belon...
Embodiment 2
[0056] In the embodiment of the present invention, the quality control condition is whether the diagnostic data conforms to the symptom description.
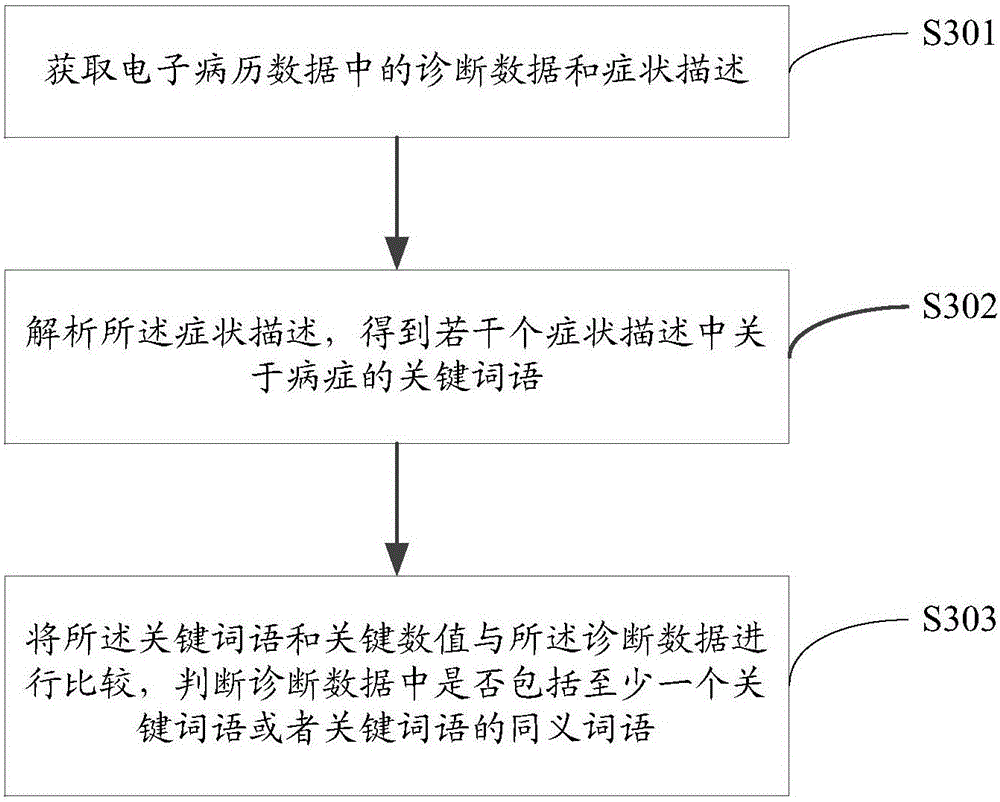
[0057] image 3 It shows the process of judging whether the electronic medical record data meets the quality control conditions provided by the embodiment of the present invention, and the details are as follows:
[0058] In step S301, the diagnostic data and symptom description in the electronic medical record data are acquired.
[0059] In step S302, the symptom descriptions are analyzed to obtain key words about the disease in several symptom descriptions.
[0060] For example, the symptom description mainly describes the irregular menstruation of a female patient. At this time, the key words about the disease should include words such as "menstruation", "abdominal pain", and "duration".
[0061] Since the structured storage of electronic medical records is very common, the embodiment of the present invention aims at the el...
Embodiment 3
[0066] In the embodiment of the present invention, the number of the quality control conditions is two or more.
[0067] It should be applicable to the situation where multiple quality control conditions are used in the electronic medical record scoring process at the same time.
[0068] Figure 4 It shows the process of obtaining the quality score of the electronic medical record according to the judgment result and the quality control rule corresponding to the quality control condition provided by the embodiment of the present invention. The quantity of the quality control conditions is two or more. The details are as follows:
[0069] In step S401, according to the judgment result, the grade score corresponding to the quality control condition is obtained.
[0070] For example, when the quality control condition is "whether the admission record is completed within 24 hours", if the judgment result is "Yes", the grade score is "10 points", which means adding 10 points; if...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com