Bile acid-alpha-hydroxyphosphonate derivatives and synthetic method thereof
A technology of hydroxyphosphonate and derivatives, which is applied in drug combinations, steroids, antineoplastic drugs, etc., can solve the problems that have not been reported on cholic acid-α-hydroxyphosphonate derivatives, and achieve excellent targeting Effect of selectivity and antitumor activity, good amphipathicity, easy modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
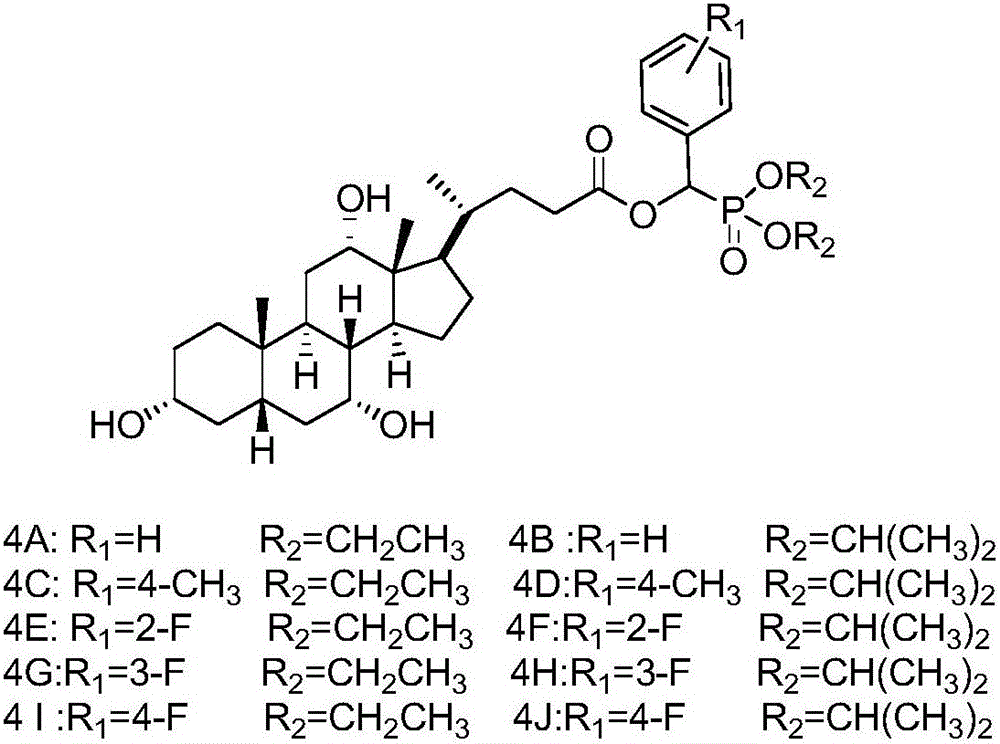
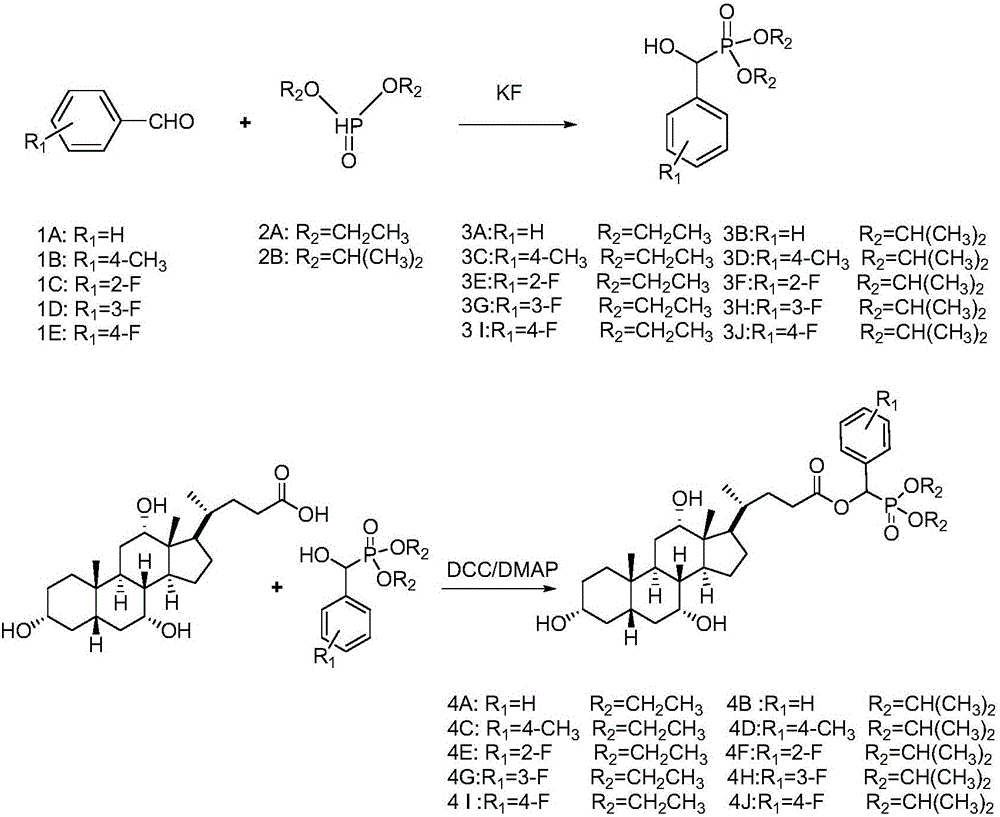
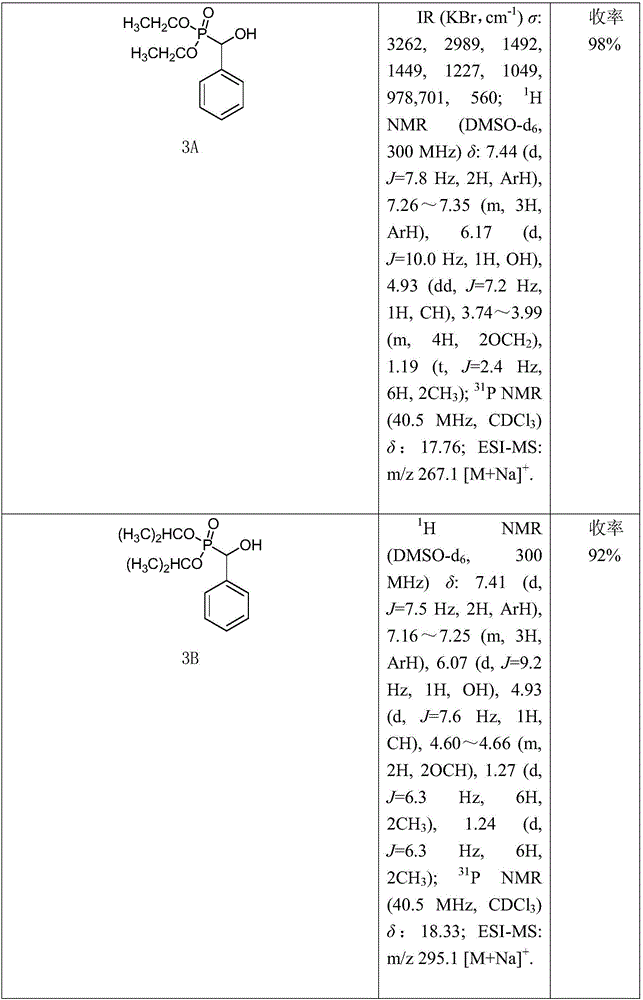
[0018] (1) Synthesis of Compound 3: Add 10mmol of Compound 2A (or 2B) to a 50mL round bottom flask, then slowly add 12mmol of Compound 1A (or 1B, 1C) dropwise, stir well, then add 1.15g of anhydrous potassium fluoride. Stir vigorously at room temperature until all the liquid in the flask turns into a white solid, add 30 mL of dichloromethane to dissolve it, filter to remove potassium fluoride, distill under reduced pressure to spin the filtrate to dryness, and recrystallize to obtain intermediate 3A compound (or 3B ~3J).
[0019] (2) Synthesis of compound 4: 6 mmol of DCC and 1 mmol of DMAP were dissolved in 10 mL of tetrahydrofuran, and set aside for later use. Add 40 mL of dried tetrahydrofuran into a 100 mL round-bottomed flask, then add 5 mmol of 3A compound (or 3B to 3J) and 6 mmol of cholic acid, and stir to dissolve it. Under an ice bath, the catalyst was slowly added dropwise into the round bottom flask. After the dropwise addition, the temperature was raised to 65° ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com