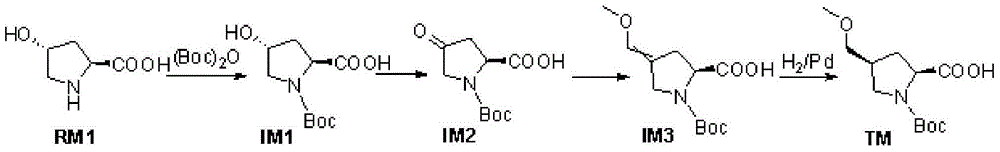(4S)-N-Boc-4-methoxymethyl-L-proline synthesis method
A technology of -n-boc-4--, -n-boc-4-, applied in the field of medicine, can solve the problems of high raw material cost and production cost, long reaction line, safety problems, etc., and achieve the reduction of raw material cost and saving Economic cost and the effect of reducing environmental pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A kind of synthetic method of (4S)-N-Boc-4--methoxymethyl-L-proline of the present embodiment comprises the following steps:
[0030] a. Using L-hydroxyproline as a starting material, the step of obtaining N-Boc-L-hydroxyproline through Boc protection;
[0031] b. Dissolve N-Boc-L-hydroxyproline in a solvent and oxidize it with TEMPO (tetramethylpiperidine nitrogen oxide) to obtain (2S)-N-Boc-4-oxopyrrolidine-2-carboxy acid step;
[0032] c. Dissolve (2S)-N-Boc-4-oxopyrrolidine-2-carboxylic acid and methoxymethyltriphenylphosphonium chloride in a solvent for wittig reaction to prepare (2S)-N-Boc - the step of 4-methoxymethylenepyrrolidine-2-carboxylic acid;
[0033] d. Dissolve (2S)-N-Boc-4-methoxymethylenepyrrolidine-2-carboxylic acid and tert-butylamine in water to prepare (4S)-N-Boc-4--methoxymethyl base-L-proline; its synthetic route is:
[0034]
[0035] In this example, in step a, dissolve L-hydroxyproline in water to adjust the pH to 8-9, then heat to 20-2...
Embodiment 1
[0046]In a 2L reaction flask, add 131.13g (1mol) L-hydroxyproline and 500mL water, stir to dissolve it; then add about 240g saturated potassium carbonate solution to adjust the pH to 8-9. The reaction solution was heated to 23°C, and 218.25g (1mol) (Boc) was added dropwise 2 O in 500 mL THF. After the dropwise addition was completed, the reaction was incubated at 23° C. for 18 hours. After the reaction is completed, distill under reduced pressure to remove THF in the system, extract the water phase with 2×250mL methyl tert-butyl ether, then cool to 3°C, adjust to PH=2~3 with 4N HCl, add solid NaCl, and use 1.1L ethyl acetate extracted 3 times. The organic phases were combined, washed with 300 mL of saturated brine, separated, the organic phase was dried over anhydrous sodium sulfate for 2 h, filtered, and the filtrate was concentrated to obtain an oil, which became a white solid after standing, weighing 230.0 g, yield 99%.
Embodiment 2
[0048] In a 2L reaction flask, add 131.24g (1mol) L-hydroxyproline and 500mL water, stir to dissolve it; then add about 240g saturated potassium carbonate solution to adjust the pH to 8-9. The reaction solution was heated to 20°C, and 218.18g (1mol) (Boc) was added dropwise 2 O solution in 500mL THF, after the dropwise addition, was incubated at 20°C for 16h. After the reaction was completed, the THF in the system was distilled off by distillation under reduced pressure. The aqueous phase was extracted with 2×250mL methyl tert-butyl ether, then cooled to 0°C, adjusted to pH=2~3 with 4N HCl, added solid NaCl, and extracted three times with 1.1L ethyl acetate. The organic phases were combined, washed with 300 mL of saturated brine, separated, the organic phase was dried over anhydrous sodium sulfate for 2 h, filtered, and the filtrate was concentrated to obtain an oil, which became a white solid after standing, weighing 223.1 g, yield 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com