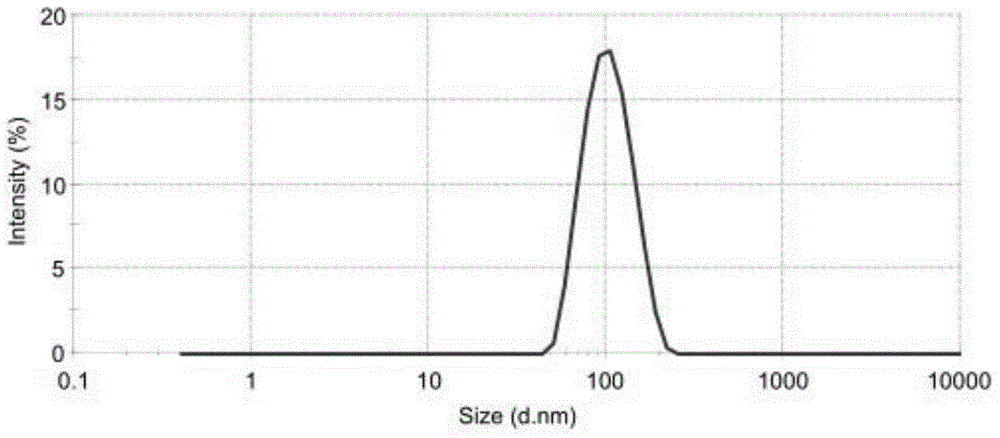
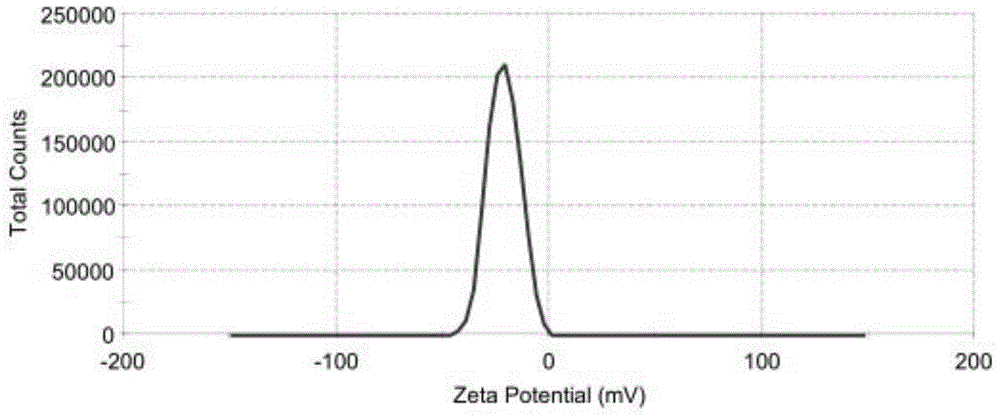
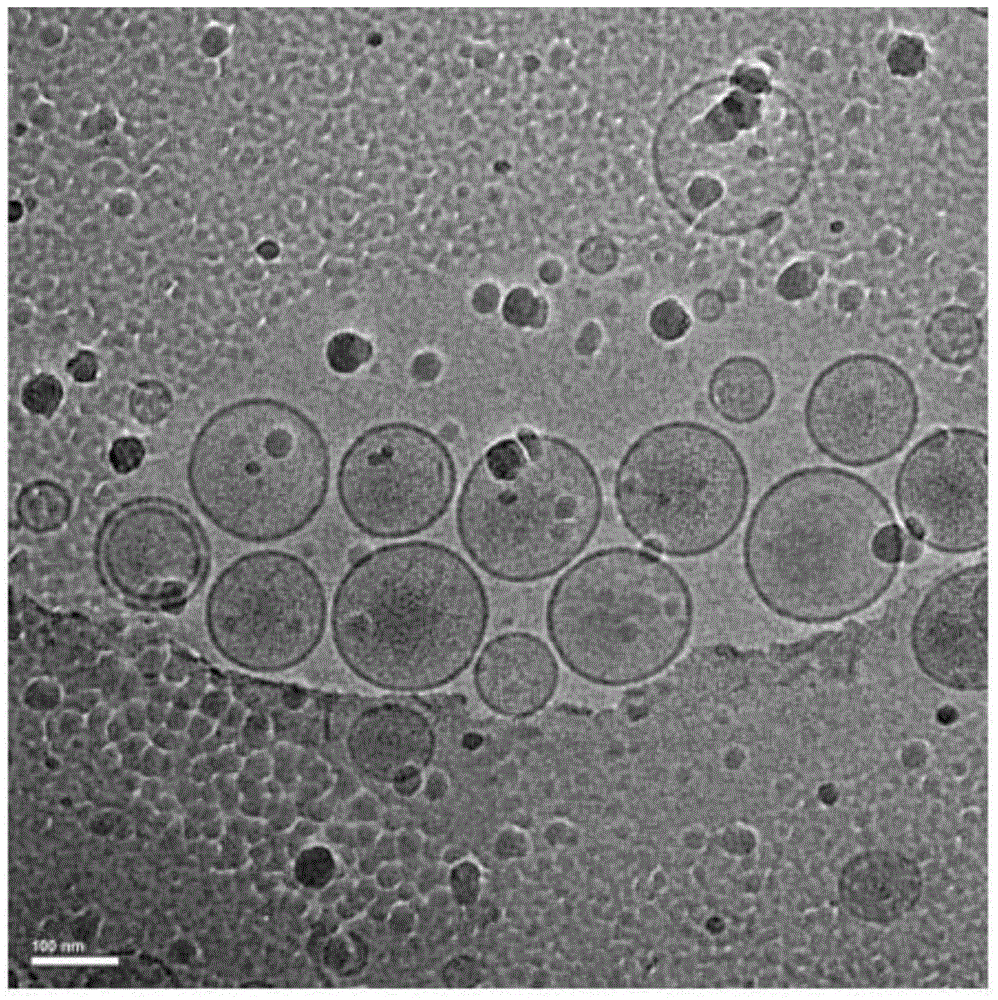
Irinotecan hydrochloride liposome pharmaceutical composition and preparation method thereof
A technology of irinotecan hydrochloride and its composition, which is applied in the field of liposome composition and its preparation, can solve the problems of instability and easy degradation, and achieve the effect of stable drug loading and high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 irinotecan hydrochloride liposome injection
[0042] Table 1: The ratio of each prescription of irinotecan hydrochloride liposome injection
[0043]
[0044] Preparation:
[0045] Put the prescribed amount of hydrogenated soybean phosphatidylcholine, distearoylphosphatidylcholine, cholesterol, and DSPE-mPEG2000 in a 500ml round-bottomed flask, add 200ml of absolute ethanol to dissolve, and reduce the temperature on a rotary evaporator at 55-60°C. Ethanol is removed by pressure steaming, so that the lipid forms a uniform lipid film at the bottom of the flask, and the film is washed by rotation with 100ml0.25mol / L ammonium sulfate aqueous solution, and then the granules are extruded successively through 0.4, 0.2, 0.1 μm by a homogenizer (each 3 times), a tangential flow ultrafiltration device was used to remove unwrapped ammonium sulfate, and water for injection was constantly replenished during the ultrafiltration process to obtain blank liposomes.
[0...
Embodiment 2
[0060] Embodiment 2 irinotecan hydrochloride liposome injection
[0061] Table 4: Prescription ratio of irinotecan hydrochloride liposome injection
[0062] Irinotecan Hydrochloride
[0063] Preparation:
[0064] Weigh the prescribed amount of hydrogenated soybean phosphatidylcholine, distearoylphosphatidylcholine, cholesterol, and DSPE-mPEG2000, put them in a 500ml round bottom flask, add 200ml of absolute ethanol to dissolve, and reduce the temperature on a rotary evaporator at 55-60°C. Ethanol was evaporated under pressure to make the lipid form a uniform lipid film at the bottom of the flask, and the film was washed with 100ml of 0.25mol / L ammonium sulfate aqueous solution, and then the whole grain was extruded through a homogenizer at 0.4, 0.2, and 0.1 μm in sequence ( Each time 3 times), a tangential flow ultrafiltration device was used to remove unwrapped ammonium sulfate, and water for injection was continuously replenished during the ultrafiltration process...
Embodiment 3
[0070] Table 6: Prescription ratio of irinotecan hydrochloride liposome injection
[0071] Irinotecan Hydrochloride
0.4g
Hydrogenated Soy Phosphatidylcholine
1.44g
Distearoylphosphatidylcholine
0.48g
cholesterol
0.5g
DSPE-mPEG3350
0.4g
5g
10g
0.38g
0.65g
Water for Injection
Add to required volume
[0072] Preparation:
[0073] Weigh the prescribed amount of hydrogenated soybean phosphatidylcholine, distearoylphosphatidylcholine, cholesterol, DSPE-mPEG3350 and add 50ml of absolute ethanol to dissolve, then inject 100ml of 0.25mol / L ammonium sulfate kept in a 60°C water bath through a peristaltic pump in the aqueous solution, and then through a homogenizer to extrude granules through 0.4, 0.2, and 0.1 μm successively (3 times each). A tangential flow ultrafiltration device is used to r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com