miRNA biomarker and detection kit used for renal cancer diagnosis
A technology of biomarkers and kits, which is applied in the determination/testing of microorganisms, DNA/RNA fragments, recombinant DNA technology, etc., and can solve problems such as microRNA biomarkers that have not been found in kidney cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
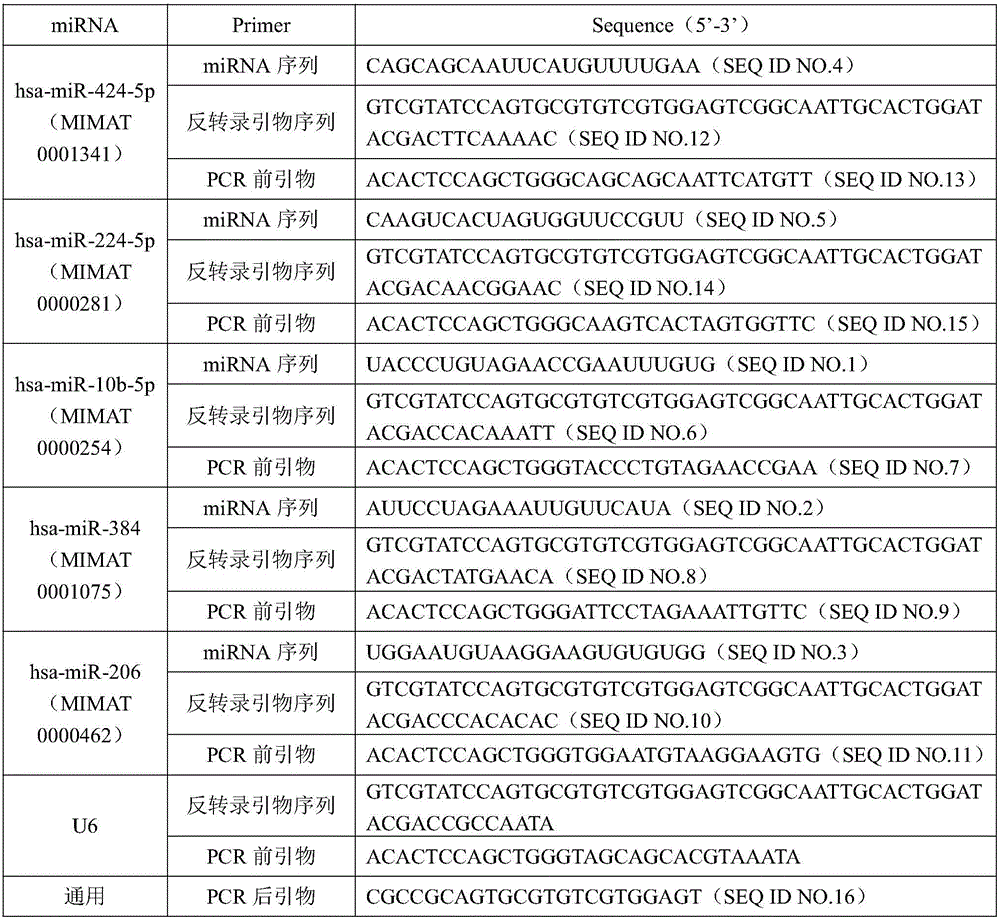
[0015] Example 1: Screening of differential miRNAs in renal cancer tissues
[0016] 1 Objects and methods
[0017] 1.1 Specimen source
[0018] After obtaining the patient’s informed consent, 8 pairs of specimens were collected from Jiangsu Provincial Hospital of Integrated Traditional Chinese and Western Medicine from April 2013 to April 2014, which were confirmed by pathology as kidney cancer patients after surgery, including cancer tissue specimens and paired specimens with a distance of more than 3 cm from the cancer tissue. Paracancerous tissue, all patients had not received chemotherapy and radiotherapy before operation.
[0019] 1.2 Main instruments and equipment
[0020] Desktop centrifuge: Eppendorf Minispin (USA) Eppendorfcentrifuge5810R (USA)
[0021] Nucleic acid concentrator: Eppendorfconcentrator5301 (USA)
[0022] Ultraviolet spectrophotometer: DU640, balance (Beckman company product)
[0023] Ultraviolet crosslinking instrument: GSGENELINKERUVChamber (prod...
Embodiment 2
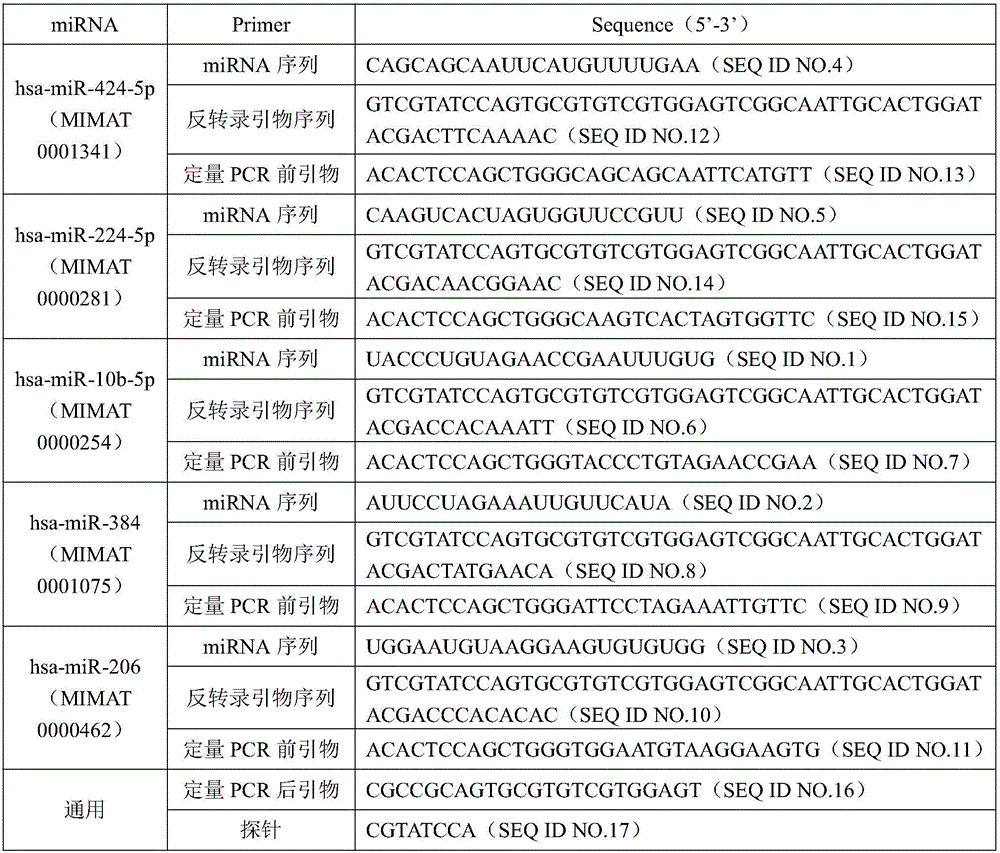
[0094] Example 2: Serological Analysis of Differential miRNAs in Renal Cancer Tissues
[0095] 1 Objects and methods
[0096] 1.1 Specimen source
[0097] After obtaining the informed consent of the patients, 165 patients with pathologically diagnosed kidney cancer and 120 healthy people with early morning fasting venous blood (6ml) were collected from Jiangsu Provincial Hospital of Integrated Traditional Chinese and Western Medicine from May 2013 to August 2014 as a control. All kidney cancer patients were diagnosed for the first time, and had not undergone surgery, radiotherapy or chemotherapy before blood collection. The 120 healthy control groups were age-matched healthy people without malignant tumors or other diseases.
[0098] 1.2 Serum sample collection and processing
[0099] Draw 6ml of fasting venous blood in the morning and put it in a tube without anticoagulant, let it stand for 30 minutes, centrifuge at 1300g (or 4000rpm) at 4°C for 15 minutes, take the upper ...
Embodiment 3
[0149] Embodiment 3: receiver operating characteristic curve (ROC) analysis
[0150] A ROC curve was constructed to compare the diagnostic ability of five serum miRNAs in distinguishing RCC patients from healthy controls. The areas under the ROC curve (AUC) of the five miRNAs were: hsa-miR-10b-5p, 0.796 (95% confidence interval: 0.760-0.920); hsa-miR-384, 0.789 (95% confidence interval: 0.736-0.902); hsa-miR-206, 0.774 (95% confidence interval: 0.734-0.894); hsa-miR-424-5p, 0.793 (95% confidence interval: 0.765-0.920); hsa-miR-224-5p, 0.732 (95% Confidence interval: 0.644-0.832). Under the optimal cutoff value, the sensitivity and specificity of miRNAs are as follows: hsa-miR-10b-5p, 83.3% and 82.7%, respectively; hsa-miR-384, 64.8% and 94.2%, respectively; hsa-miR- 206, respectively, 90.7% and 61.5%; hsa-miR-424-5p, respectively, 70.4% and 82.5%; hsa-miR-224-5p, respectively, 67.3% and 74.1%. The combined AUC of these five miRNAs can reach 0.992, and the sensitivity and sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com