Application of NIK protein kinase inhibitor in serving as medicine for treating liver diseases
A protein kinase inhibitor, drug technology, applied in the application field of NIK protein kinase inhibitor, acute liver injury, treatment of liver inflammation, liver fibrosis and liver cirrhosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
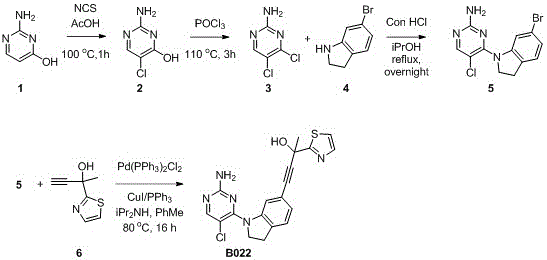
[0025] Example 1: Synthesis of NIK inhibitor B022.
[0026] Materials and Methods:
[0027] 2-Amino-5-chloropyrimidin-4-ol (2)
[0028] At room temperature, 2-aminopyrimidin-4-ol (1 g, 9.1 mmol), acetic acid (10 mL) and NCS (1.32 g, 10 mmol) were successively added into a round bottom flask, and reacted at 100oC for 1 h. The acetic acid was removed by rotary evaporation, and water (20 mL) was added. The precipitate was filtered off and dried under vacuum overnight. 720 mg of the target compound was obtained, and the yield was 54%. 1HNMR (DMSO-d6, 300MHz): 7.77(s, 1H), 6.83(s, 2H). ESI-MS theoretical calculation value C4H5ClN3O[M+H]+=146.01, experimental measurement: 145.75.
[0029] 4,5-dichloropyrimidin-2-amine (3)
[0030] 2-Amino-5-chloropyrimidin-4-ol (5.16g, 36.5mmol) and POCl3 (40mL, excess) were successively added to a round bottom flask, and reacted at 110oC for 3h. Add saturated NaHCO3 aqueous solution to adjust pH>8. The mixture was filtered and the filtrate w...
Embodiment 2
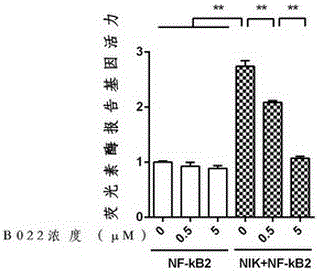
[0036] Example 2: The effect of B022 on the expression of NF-kB2 (p52) induced by NIK in Q293T cells was detected by luciferase reporter gene assay.
[0037] Materials and Methods:
[0038] Q293T cells
[0039] pShuttletrackNIK plasmid
[0040] pGL3NF-kB2 plasmid (with luciferase reporter gene)
[0041] βGal plasmid
[0042] A total of 6 experimental conditions were designed, namely:
[0043] (1) Double transfection of βGal plasmid and NF-kB2 plasmid, the concentration of B022 was 0 μM;
[0044] (2) Double transfection of βGal plasmid and NF-kB2 plasmid, the concentration of B022 was 0.5 μM;
[0045] (3) Double transfection of βGal plasmid and NF-kB2 plasmid, B022 concentration was 5 μM;
[0046] (4) Triple transfection with βGal plasmid, NIK plasmid and NF-kB2 plasmid, B022 concentration is 0 μM;
[0047] (5) Triple transfection with βGal plasmid, NIK plasmid and NF-kB2 plasmid, B022 concentration is 0.5 μM;
[0048] (6) Triple transfection with βGal plasmid, NIK plas...
Embodiment 3
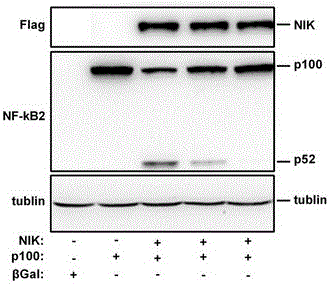
[0052] Example 3: Western blot detection of the effect of B022 on the expression of NF-kB2 (p52) induced by NIK in Hep1 cells.
[0053] Hep1 cells were infected with βGal, NIK and p100 adenovirus single, double or triple, and the expression of NF-kB2 (p52) was detected by western blot.
[0054] Materials and Methods:
[0055] Hep1 cells
[0056] βGal, NIK and p100 adenoviruses
[0057] B022
[0058] A total of 5 experimental conditions were designed, namely:
[0059] (1) βGal single infection;
[0060] (2) p100 single infection;
[0061] (3) p100+NIK double infection, the content of NIK virus is 1 / 10 of p100, and the concentration of B022 is 0 μM;
[0062] (4) p100+NIK double infection, the content of NIK virus is 1 / 10 of p100, and the concentration of B022 is 0.5 μM;
[0063] (5) p100+NIK double infection, the content of NIK virus is 1 / 10 of p100, and the concentration of B022 is 5 μM;
[0064] Specific experimental steps:
[0065] (1) Preparation of Hep1 cells: spre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com