a cl - Synergistic activation of PMS/cnt to degrade azo dyes
A technology of azo dyes and pH value, applied in chemical instruments and methods, water/sludge/sewage treatment, water pollutants, etc., can solve the problems of secondary pollution, complicated operation, high energy consumption, etc., and achieve strong oxidation ability , Reduce salt content, wide pH range effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 0.025 g CNT, 7 mL of 57 mmol L -1 PMS and 238 mL of distilled water were added to a 250 mL wide-mouth Erlenmeyer flask, then placed on a stirrer and stirred evenly, and 2 mol L -1 Sodium hydroxide solution to adjust the pH of the system to 7, then add 5 ml of 4 mmol L -1 OG. The reaction was uniformly stirred at room temperature for 45 min, and the removal of the azo dye OG by CNT-activated PMS alone was completed. In the present embodiment, the removal rate of OG reached 85% in 45 min, wherein the concentration of azo dye OG was measured using a UV-Vis spectrophotometer.
Embodiment 2
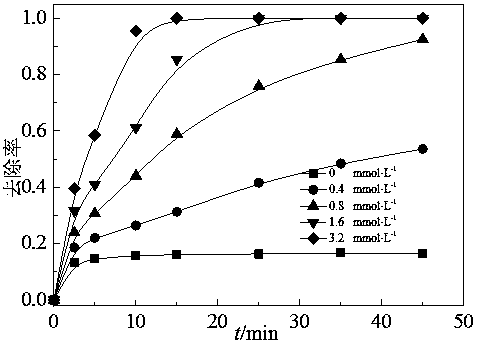
[0029] 25 mL of 500 mmol·L -1 NaCl, 0.025 g CNT and 0 mL, 1.75 mL, 3.5 mL, 7 mL and 14 mL of 57 mmol·L -1PMS was added to 250 mL wide-mouth conical flasks respectively, and then a certain amount of distilled water was added to reach a total solution of 245 mL, placed on a stirrer and stirred evenly, and 2 mol L -1 Sodium hydroxide solution was used to adjust the pH of the system to 7, and then 5 ml of 4 mmol·L -1 OG. The reaction was evenly stirred at room temperature for 45 min, and the Cl - Promote the removal of azo dye OG by CNT / PMS system. see test results figure 1 , The removal rate of OG in the present embodiment is 17%, 54%, 93%, 100%, 100% respectively.
Embodiment 3
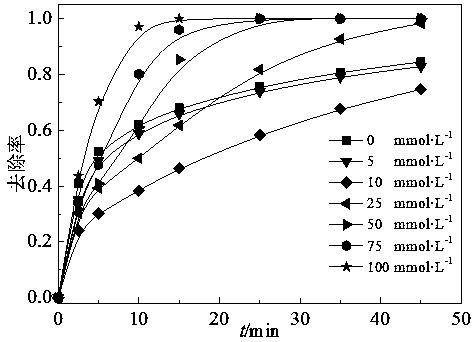
[0031] 7 mL of 57 mmol·L -1 PMS, 25 mL of 500 mmol L -1 NaCl and 213 mL of distilled water were added to a 250 mL wide-mouth Erlenmeyer flask, then placed on a stirrer and stirred evenly, and 2 mol L -1 Sodium hydroxide solution to adjust the pH of the system to 7, then add 5 ml of 4 mmol L -1 OG. The reaction was uniformly stirred at room temperature for 45 min, and the removal of the azo dye OG by the action of NaCl and PMS alone was completed. see test results figure 2 , the removal rate of OG in 45 min reaches 82% in the present embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com