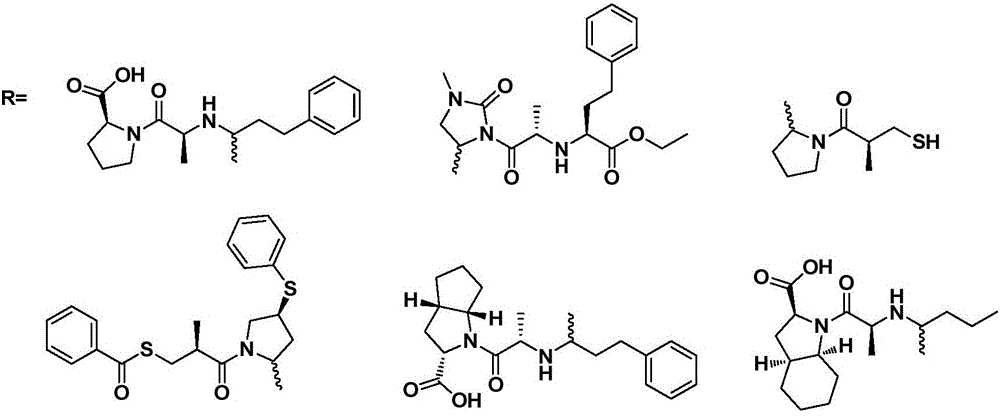
Preparation and medical application of ACEI (angiotensin converting enzynme inhibitor)-type berberine conjugate
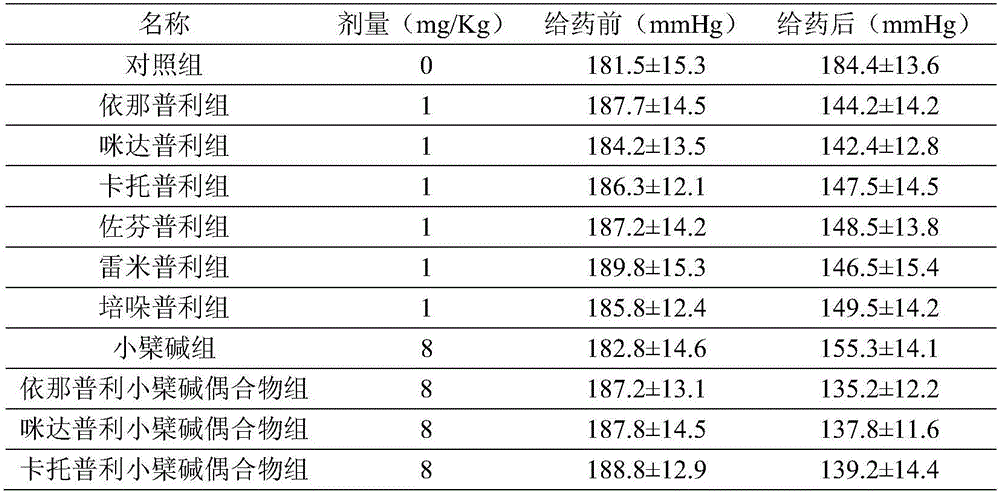
The technology of a conjugate, berberine hydrochloride, is applied in blood pressure regulating products. In the field of treatment of high blood pressure, the Prism-like berberine conjugate can solve the problems of low fat solubility, poor water solubility, and malabsorption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of Enalapril and Berberine Conjugates
[0021] Take 3.7g of berberine hydrochloride, put it into a 500ml three-neck flask, add 100ml of ethanol, adjust the pH to 7-8 with 3mol / l sodium hydroxide, raise the temperature to 60-70°C, stir to dissolve, then add 3.5g of enalapril Keep stirring at this temperature for 1-5 hours, cool down to room temperature, crystallize, filter, and dry to obtain 5.7 g of enalapril berberine conjugate with a yield of 84%.
Embodiment 2
[0023] Synthesis of Enalapril and Berberine Conjugates
[0024] Take 3.7g of berberine hydrochloride, put it into a 500ml three-neck flask, add 100ml of ethanol, adjust the pH to 7-8 with 3mol / l potassium hydroxide, raise the temperature to 60-70°C, stir to dissolve, then add 3.7g of enalapril Keep stirring at this temperature for 1-5 hours, cool down to room temperature, crystallize, filter, and dry to obtain 6.1 g of enalapril berberine conjugate with a yield of 90%. ESI-MS (M + +H)m / zcalcdforC 20 h 18 NO 4 + 337.12found337.21; ESI-MS (M + +H)m / zcalcdforC 18 h 23 N 2 o 5 348.16found348.34.
Embodiment 3
[0026] Synthesis of imidapril and berberine conjugates
[0027] Take 3.7g of berberine hydrochloride, put it into a 500ml three-necked flask, add 100ml of ethanol, adjust the pH to 7-8 with 3mol / l sodium hydroxide, raise the temperature to 60-70°C, stir to dissolve, then add 4.3g of Midap Keep stirring at this temperature for 1-3 hours, cool down to room temperature, crystallize, filter, and dry to obtain 6.5 g of imidapril berberine conjugate with a yield of 88%. ESI-MS (M + +H)m / zcalcdforC 20 h 18 NO 4 + 337.12found337.16; ESI-MS (M + +H)m / zcalcdforC 20 h 26 N 3 o 6 405.19 found 405.27.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com