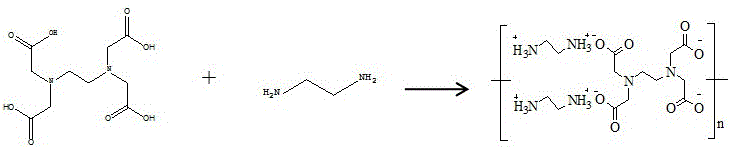Preparation method of fluorescent ammonia carboxylate
An ammonium carboxylate and fluorescence technology is applied in the field of preparing ammonium carboxylate with fluorescence, and achieves the effects of easy purification, simple preparation method and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of fluorescent ethylenediaminetetraacetic acid-ethylenediammonium salt, the steps are as follows:
[0031] 1) Dissolve 1g of ethylenediaminetetraacetic acid and 0.4109g of ethylenediamine in 5ml of distilled water, place it in a solution vacuum bottle under the protection of nitrogen, and oscillate ultrasonically for 10 minutes until all the raw materials are dissolved and become a colorless and transparent solution. After stirring at 30°C for 12 hours, the solution turned into a brown transparent liquid, and mixture Ⅰ was obtained;
[0032] 2) After the mixture I was washed three times with petroleum ether, water and petroleum ether were removed by rotary evaporation under reduced pressure to obtain a transparent solid, and a fluorescent ethylenediaminetetraacetic acid-ethylenediammonium salt was obtained.
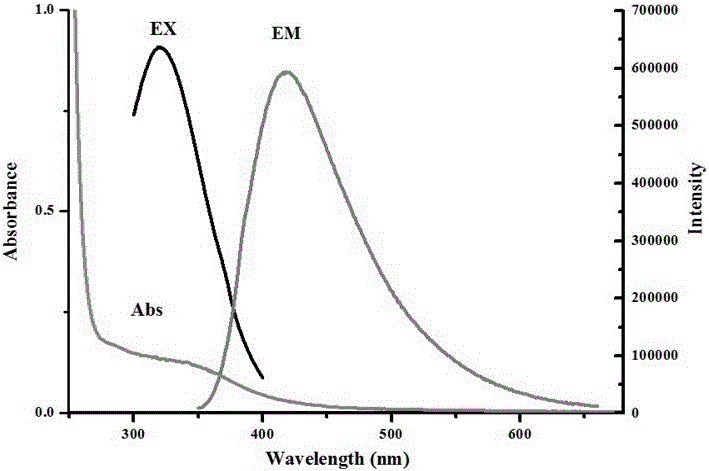
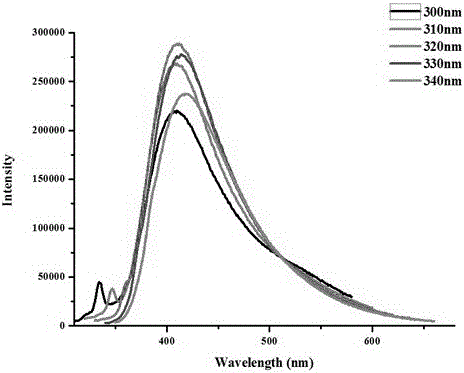
[0033] Figure 1~Figure 3 The fluorescence properties of ethylenediaminetetraacetic acid-ethylenediammonium salt were characterized. figure 1 It ...
Embodiment 2
[0039] The preparation of fluorescent ethylenediaminetetraacetic acid-ethylenediammonium carboxylate ammonium salt, the steps are as follows:
[0040] 1. Dissolve 1g of ethylenediaminetetraacetic acid and 0.4109g of ethylenediamine in 5ml of distilled water, place it in a solution vacuum bottle under the protection of nitrogen, and oscillate ultrasonically until the raw materials are completely dissolved and become a colorless and transparent solution. Place the device at 0 Stirring at ℃ for 36 hours, the solution turned into a brown transparent liquid, and mixture Ⅰ was obtained;
[0041] 2. After the mixture I was washed three times with petroleum ether, water and petroleum ether were removed by rotary evaporation under reduced pressure to obtain a transparent solid, and a fluorescent ammonium carboxylate was obtained.
[0042] The characterization of the product obtained is similar to Example 1.
Embodiment 3
[0044] The preparation of fluorescent ethylenediaminetetraacetic acid-ethylamine carboxylate ammonium salt, the steps are as follows:
[0045] 1. Dissolve 1g of ethylenediaminetetraacetic acid and 0.616g of ethylamine in 5ml of distilled water, place it in a solution vacuum bottle under the protection of nitrogen, and oscillate ultrasonically until all the raw materials are dissolved and become a colorless and transparent solution. Place the device at 30°C Stirring in the environment for 12h, the solution turned into a brown transparent liquid, and the mixture Ⅰ was obtained;
[0046] 2. After the mixture I was washed three times with petroleum ether, water and petroleum ether were removed by rotary evaporation under reduced pressure to obtain a transparent solid, and a fluorescent ammonium carboxylate was obtained.
[0047] Its fluorescent properties are similar to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com