Helicene diphenol hydride, method for preparing same and application of helicene diphenol hydride
A technology for hydrogenating helicene diphenols and helicene diphenols, which is applied in the directions of organic chemistry methods, carbon-based compound preparation, chemical instruments and methods, etc. Symmetrical catalytic effect, correct structure, high product yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
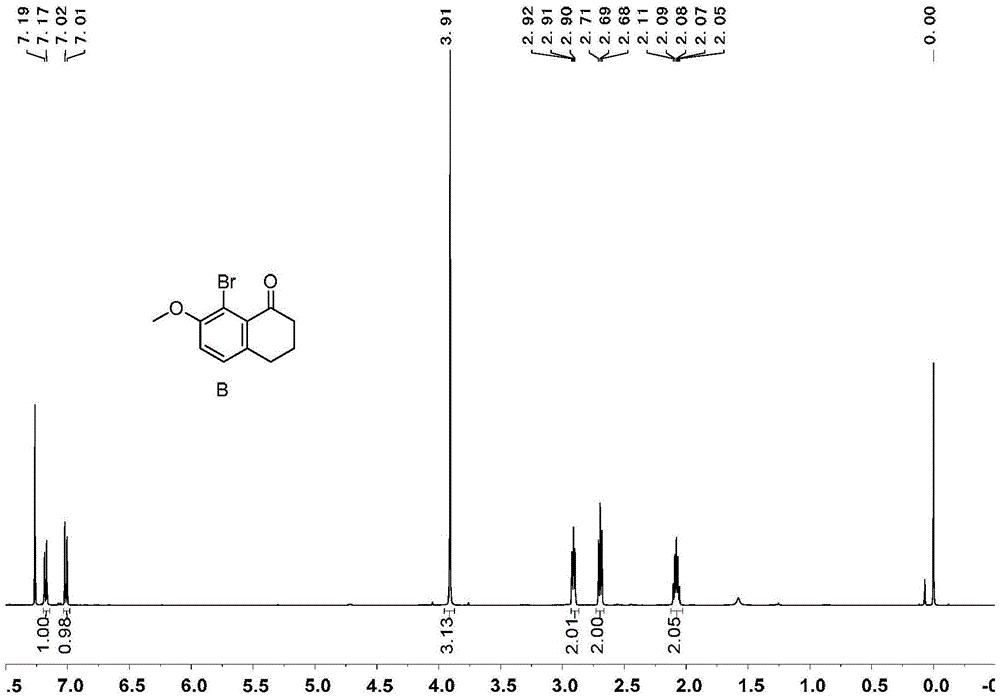
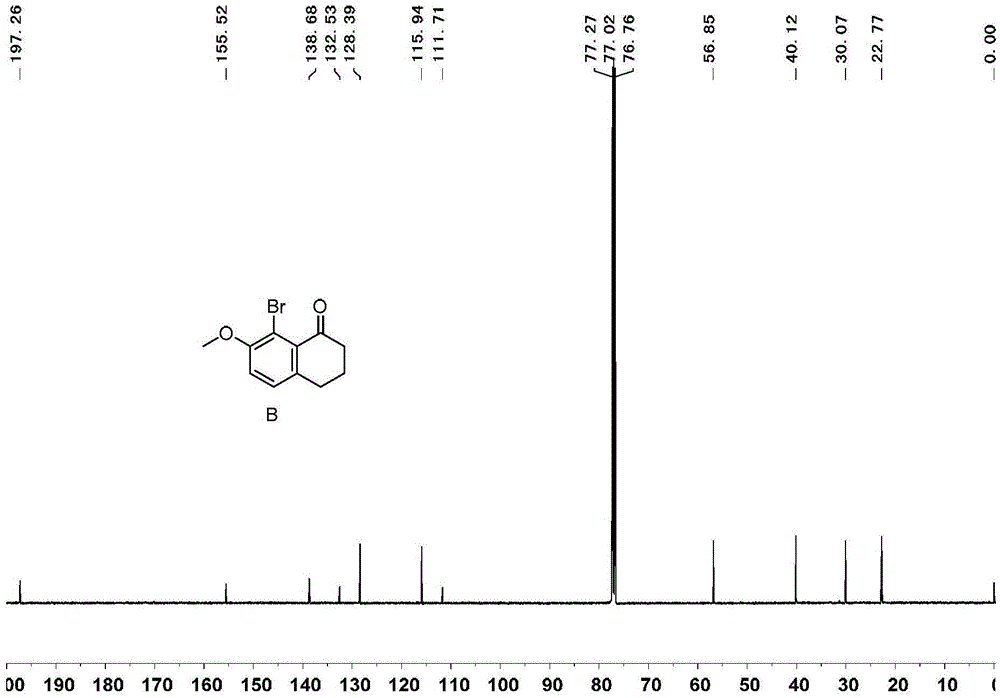
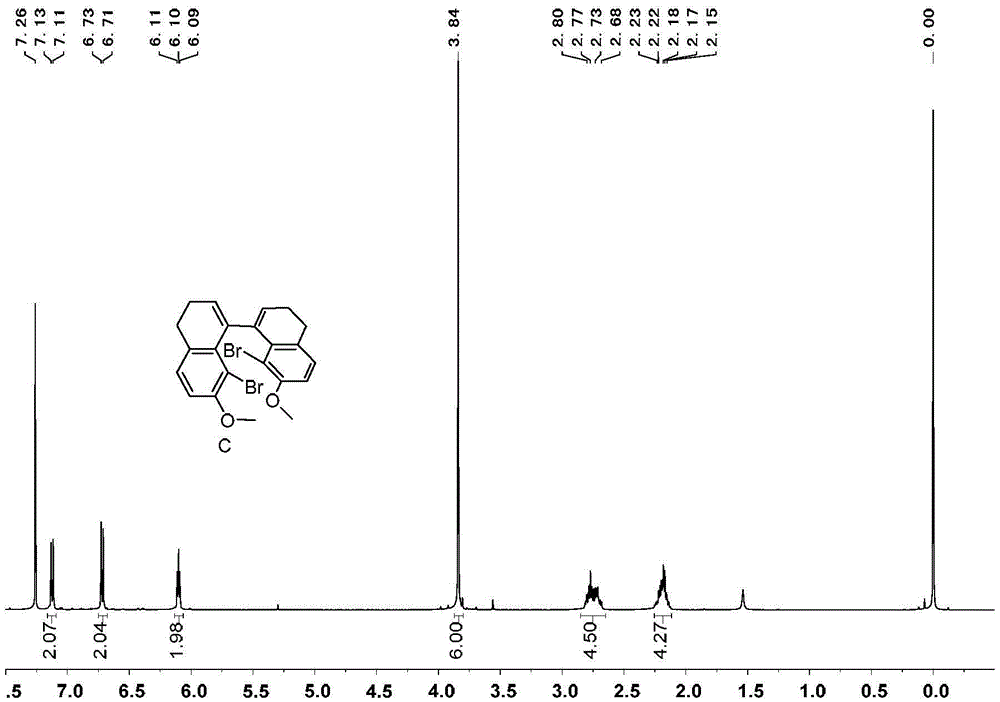
[0071] Embodiment 1, the synthesis of 1,1 '-diphenyl [5] helical diphenol
[0072] 1,1'-diphenyl[5]helicenediol is synthesized according to the following reaction scheme:
[0073]
[0074] 1) Add 99g of tetralone, 100g of N-bromosuccinimide, 4g of ferric chloride and 500mL of acetonitrile in sequence in a 1000mL round bottom flask, react at room temperature for 4 hours, add 300mL of water to the reaction system, and precipitate a white solid 143 g of product B, greater than 99% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com