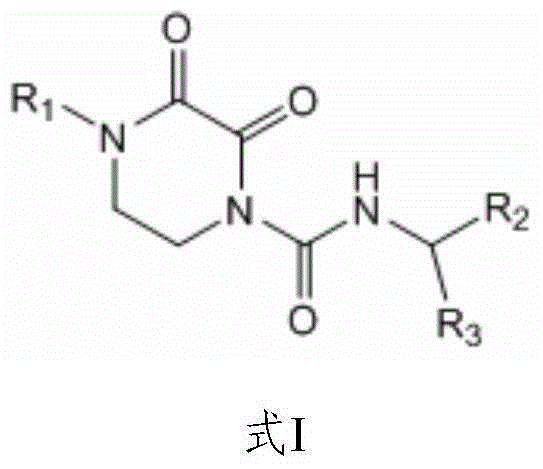
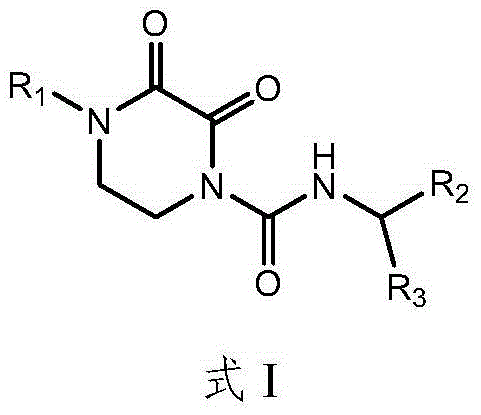
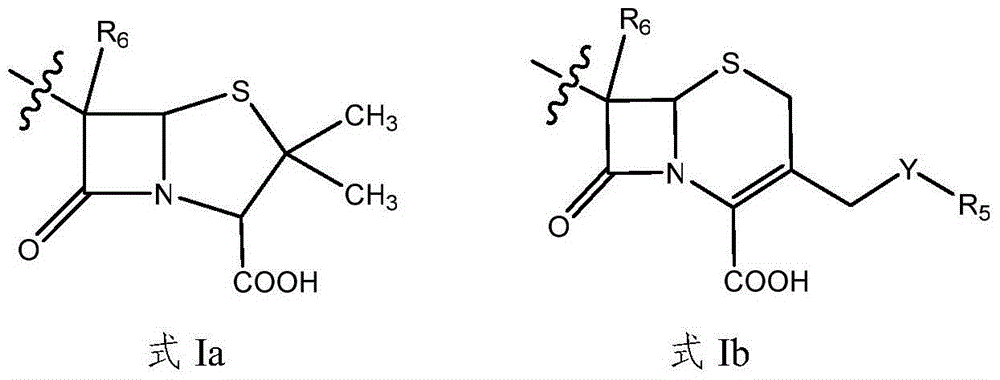
New application of oxypiperazine acidamide compound
A technology of oxopiperazine amides and compounds, which is applied in the new application field of inhibiting PLK1 activity, can solve the problems of predicting PLK1 inhibitors, raw material drug quality control is immature, and has no obvious regularity, and achieves less toxic and side effects and better efficacy Precise, easy-to-obtain results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Inhibitory effect of oxopiperazine amides on PLK1 kinase
[0059] 1. Test substance: compounds represented by formula II to formula VIII of the present invention, amoxicillin, cefuroxime, penicillin, sulbactam, clavulanic acid, their hydrates and salts, and related compositions, etc.
[0060] 2. Test method:
[0061] 1) Preparation of each group of analyte solutions: DMSO was dissolved in 20 nM HEPES aqueous solution to form a DMSO / HEPES solution with a concentration of 20%. The test substances were divided into groups (multiple control groups, individual groups, combined groups), and the test substances of each group were respectively taken and dissolved in 20% DMSO / HEPES to obtain the solutions of the test substances of each group for use. The grouping situation and the highest concentration of the analyte in each group of the analyte solution obtained (for the joint group, it refers to the concentration of the compound shown in formula II to formula VIII i...
Embodiment 2
[0076] Example 2 Inhibitory effect of oxopiperazine amides on tumors
[0077] Taking prostate cancer and ovarian cancer as examples below, the therapeutic effects of the oxopiperazine amides compounds of the present invention on cell proliferation diseases, especially tumors will be further illustrated.
[0078] 1. Treatment of the object to be tested:
[0079] Test substances: compounds represented by formula II to formula VIII in the present invention, amoxicillin, paclitaxel, sulbactam, their salts and hydrates, and related compositions, etc.
[0080] Solution preparation: divide the test substance into groups (multiple control groups, separate groups, combined groups), take the test substance in each group respectively, first add 1.23ml castor oil, shake well to dissolve, then add 1.23ml absolute ethanol , Shake well to dissolve, and prepare mother solutions of each group of analytes. Grouping situation and the concentration of the analyte in each group of analyte mother...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com