Lipoic acid injection liquid and preparation method thereof
A technology of lipoic acid and injection, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., and can solve problems such as allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
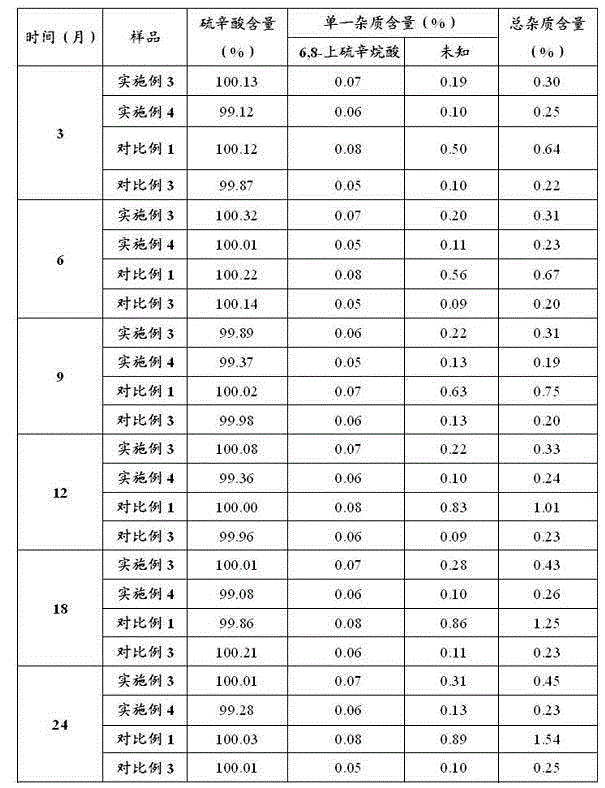
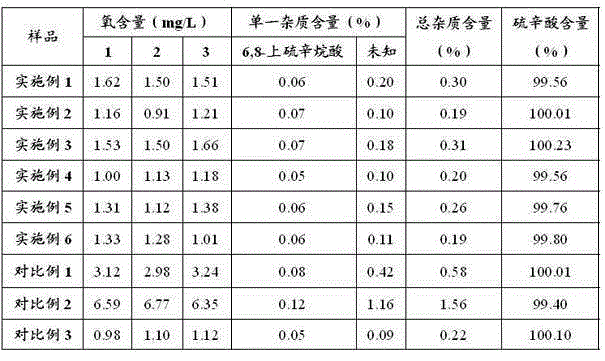
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of Lipoic Acid Injection (10L)
[0030]Add 300g of tromethamine into 6000ml of water for injection, the temperature of the water for injection is 29°C, stir and mix evenly. Add 250g lipoic acid to the above solution and mix well. Use an ultrafiltration column with a molecular weight cut-off of 5K to remove pyrogens, measure the concentration of the sample, and calculate the constant volume. Dilute to the final volume with water for injection, filter the sample after mixing evenly, pass helium gas into the bottom of the sample to remove oxygen, keep the sample solution stirring continuously, the stirring speed is subject to the vortex generated by the liquid surface, and measure the oxygen content of the sample solution to be 0.21mg / L. Fill and sterilize under the protection of helium. Before filling, the liquid is filled with helium and deoxygenated so that the oxygen content is 0.20mg / L, and the stirring speed is subject to the vortex gene...
Embodiment 2
[0031] Embodiment 2: Preparation of Lipoic Acid Injection (10L)
[0032] Add 200g of tromethamine into 8000ml of water for injection, the temperature of the water for injection is 25°C, stir and mix evenly. Add 250g lipoic acid to the above solution and mix well. Use an ultrafiltration column with a molecular weight cut-off of 5K to remove pyrogens, measure the concentration of the sample, and calculate the constant volume. After making up to the final volume with water for injection, filter the sample after mixing evenly, pass nitrogen gas into the bottom of the sample to remove oxygen, keep the sample solution stirring continuously, the stirring speed is based on the vortex generated by the liquid surface, and measure the oxygen content of the sample solution to be 0.19mg / L, filled and sterilized under nitrogen protection. Before filling, the liquid is filled with nitrogen and deoxygenated so that the oxygen content is 0.20mg / L, and the stirring speed is subject to the vo...
Embodiment 3
[0033] Embodiment 3: Preparation of Lipoic Acid Injection (10L)
[0034] Add 70g of ethylenediamine to 7000ml of water for injection, the temperature of the water for injection is 31°C, stir and mix evenly. Add 250g lipoic acid to the above solution and mix well. Use an ultrafiltration column with a molecular weight cut-off of 5K to remove pyrogens, measure the concentration of the sample, and calculate the constant volume. Dilute to the final volume with water for injection, filter the sample after mixing evenly, pass helium gas into the bottom of the sample to remove oxygen, keep the sample solution stirring continuously, the stirring speed is based on the vortex generated by the liquid surface, and measure the oxygen content of the sample solution to be 0.35mg / L, filled and sterilized under the protection of helium. Before filling, the liquid is filled with helium to deoxygenate to make the oxygen content 0.36mg / L, and the stirring speed is subject to the vortex generate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com