Catalyst for dehydrogenation of light alkanes to olefins
A technology of low-carbon alkanes and catalysts, which is applied in the field of catalysts and can solve problems such as poor stability and low activity of low-carbon alkanes dehydrogenation catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
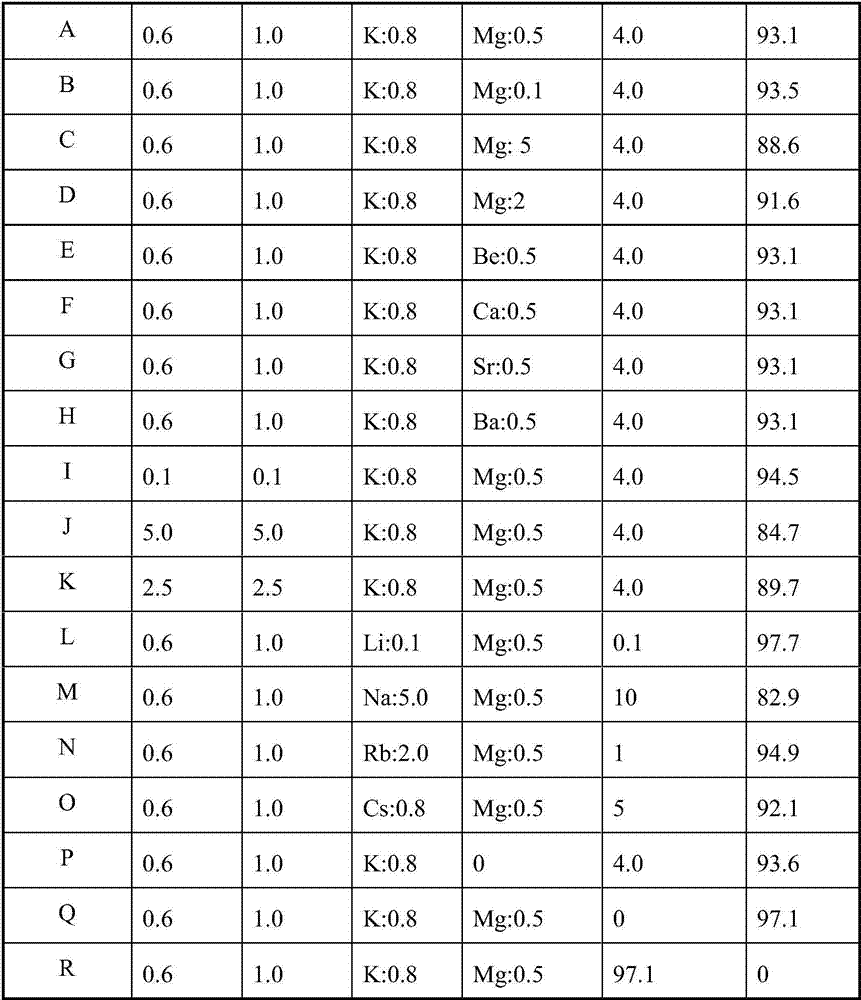
Image
Examples
Embodiment 1
[0024] Weigh 9.31g of alumina carrier and pour it into 200mL of deionized water and stir for 1 hour, weigh 1.081g of cerium ammonium nitrate and 0.212g of zirconium nitrate and dissolve them in 50mL of deionized water respectively, then mix the two evenly, pour alumina and In the mixed solution of water, after continuing to stir for 1 hour, ammonia water was slowly added dropwise under continuous stirring until the pH was 8.5. The product was aged for 2 hours, filtered and washed with 2L of water to obtain a filter cake, dried at 90°C for 16 hours, and then calcined in a muffle furnace at 580°C for 20 hours to obtain Ce 0.8 Zr 0.2 o 2 -Al 2 o 3carrier. Weigh 0.190g of stannous chloride and dissolve it in 10mL of hydrochloric acid solution, add it to the above-mentioned carrier under stirring, mix well, soak at 30°C for 12 hours, and then dry at 90°C for 16 hours to obtain the catalyst precursor, which is recorded as I. Weigh 0.159g of chloroplatinic acid, 0.207g of potass...
Embodiment 2
[0026] Weigh 9.35g of alumina carrier and pour it into 200mL of deionized water and stir for 1 hour, then weigh 1.081g of cerium ammonium nitrate and 0.212g of zirconium nitrate and dissolve them in 50mL of deionized water respectively, then mix the two evenly, pour alumina and In the mixed solution of water, after continuing to stir for 1 hour, ammonia water was slowly added dropwise under continuous stirring until the pH was 8.5. The product was aged for 2 hours, filtered and washed with 2L of water to obtain a filter cake, dried at 90°C for 16 hours, and then calcined in a muffle furnace at 580°C for 20 hours to obtain Ce 0.8 Zr 0.2 o 2 -Al 2 o 3 carrier. Weigh 0.190g of stannous chloride and dissolve it in 10mL of hydrochloric acid solution, add it to the above-mentioned carrier under stirring, mix well, soak at 30°C for 12 hours, and then dry at 90°C for 16 hours to obtain the catalyst precursor, which is recorded as I. Weigh 0.159g of chloroplatinic acid, 0.207g of ...
Embodiment 3
[0028] Weigh 8.86g of alumina carrier and pour it into 200mL of deionized water and stir for 1 hour. Weigh 1.081g of cerium ammonium nitrate and 0.212g of zirconium nitrate and dissolve them in 50mL of deionized water respectively, then mix the two evenly, pour alumina and In the mixed solution of water, after continuing to stir for 1 hour, ammonia water was slowly added dropwise under continuous stirring until the pH was 8.5. The product was aged for 2 hours, filtered and washed with 2L of water to obtain a filter cake, dried at 90°C for 16 hours, and then calcined in a muffle furnace at 580°C for 20 hours to obtain Ce 0.8 Zr 0.2 o 2 -Al 2 o 3 carrier. Weigh 0.190g of stannous chloride and dissolve it in 10mL of hydrochloric acid solution, add it to the above-mentioned carrier under stirring, mix well, soak at 30°C for 12 hours, and then dry at 90°C for 16 hours to obtain the catalyst precursor, which is recorded as I. Weigh 0.159g of chloroplatinic acid, 0.207g of potas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com