Highly active oxoaporphinoid-rhodium (III) complex, and synthetic methods and application thereof
A synthetic method, compound technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Synthesis of OGC-Rh(III) complex 1 by high-pressure solvothermal method
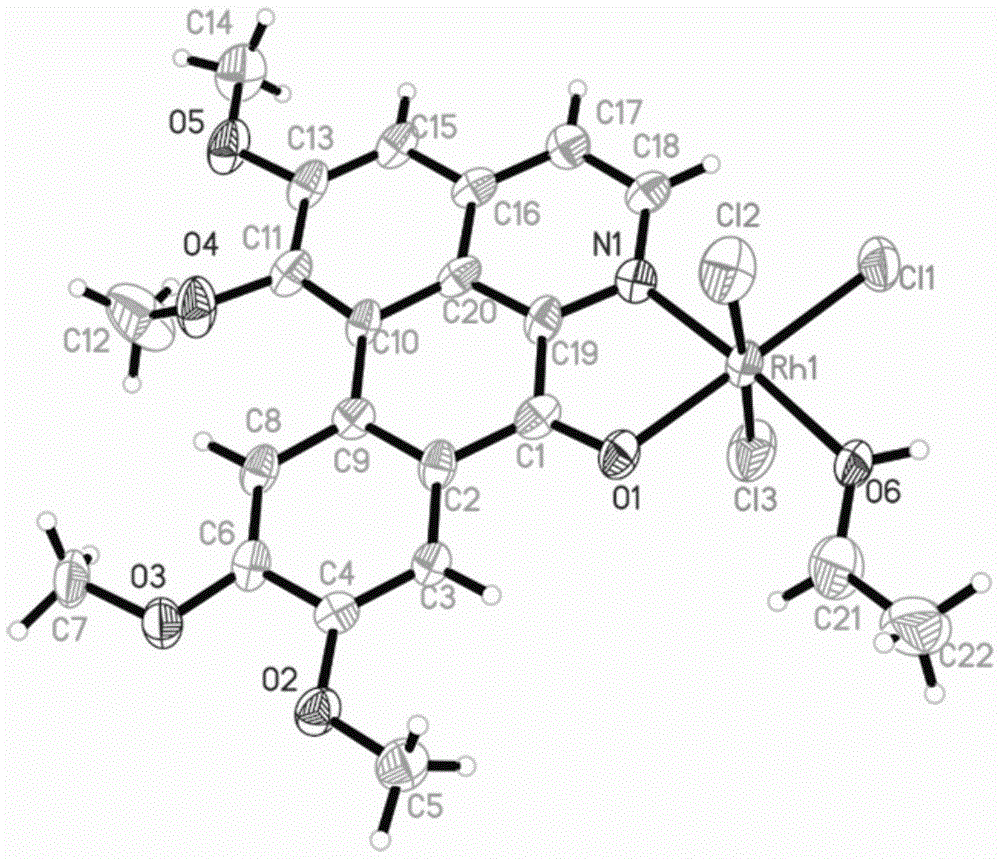
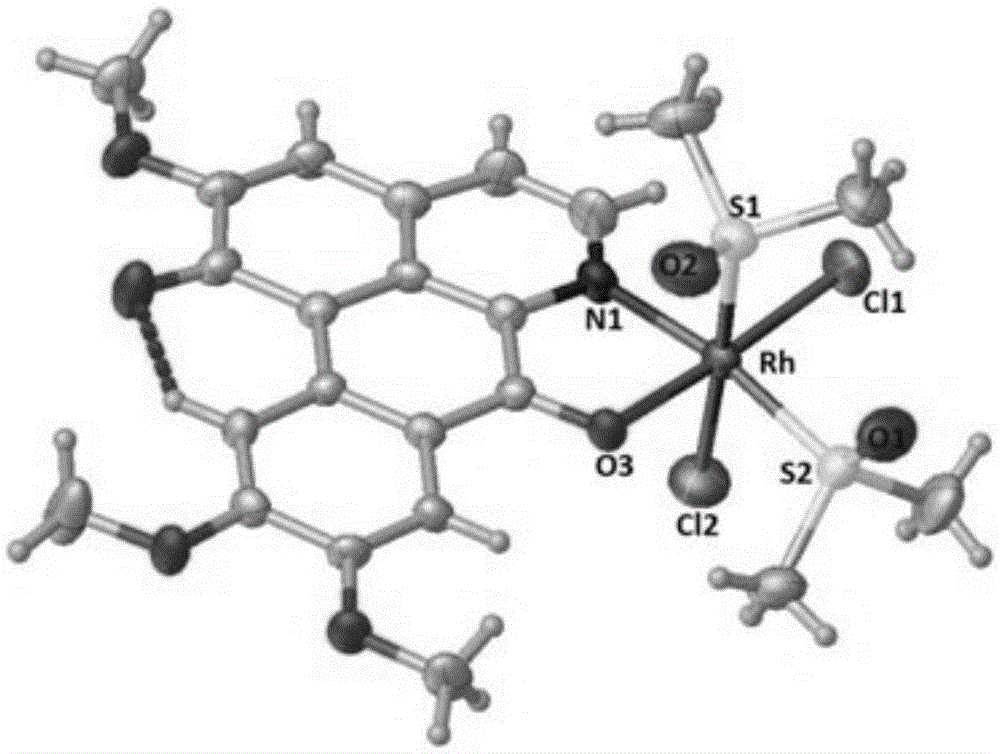
[0040] Weigh 0.1mmol OGC and add it to a 25cm Pyrex thick-walled glass tube with one end closed, add 2.0mL CH 3 CH 2 OH and 1.0mLCH 2 Cl 2 , and then weighed 0.1mmol of RhCl 3 ·nH 2 O was added to the glass tube, and after freezing with liquid nitrogen, the open end was melted and sealed under vacuum conditions. After mixing evenly, it was placed in an oven at 80°C. After 72 hours, red blocky crystals were formed in the glass tube (yield: 56%). The product was determined to be complex 1, namely [RhCl 3 (OGC)·CH 3 CH 2 OH], its crystal structure is as figure 1 shown.
Embodiment 2
[0041] Embodiment 2: Synthesis of OGC-Rh (III) complex 1 with normal pressure solution method
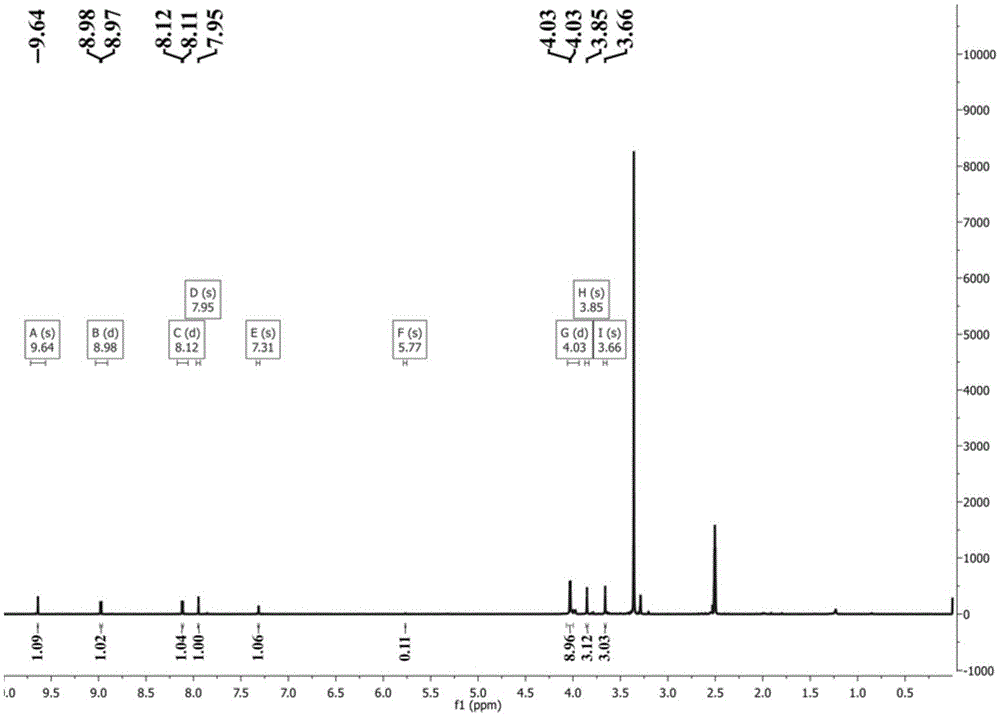
[0042] Weigh 0.28mmolRhCl 3 ·nH 2 O and 0.28mmol OGC dissolved in 10mL CH 2 Cl 2 and 10mLCH 3 CH 2 In the polar solvent composed of OH, the solution gradually changed from yellow to reddish brown; the reaction was stopped after reflux reaction at 60°C for 8 hours, and it was lowered to room temperature and left to stand overnight; filtered, the filtrate slowly evaporated at room temperature, and after two weeks, dark red square crystals It was separated out and collected after filtration (Yield: 70%). The product was determined to be complex 1 through infrared spectroscopy, ultraviolet spectroscopy, elemental analysis and electrospray mass spectrometry. 3 (OGC)·CH 3 CH 2 OH].
Embodiment 3
[0043] Embodiment 3: Synthesis of OGC-Rh (III) complex 1 with normal pressure solution method
[0044] Weigh 0.28mmolRhCl 3 ·nH 2 O and 0.28mmol OGC dissolved in 20mL CH 3 CH 2 In OH, reflux at 80°C for 2 hours to stop the reaction, drop to room temperature and let stand overnight; filter, the filtrate slowly evaporates at room temperature, dark red square crystals precipitate after two weeks, collect after filtration (Yield: 60%), The product was determined to be complex 1, namely [RhCl 3 (OGC)·CH 3 CH 2 OH].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com