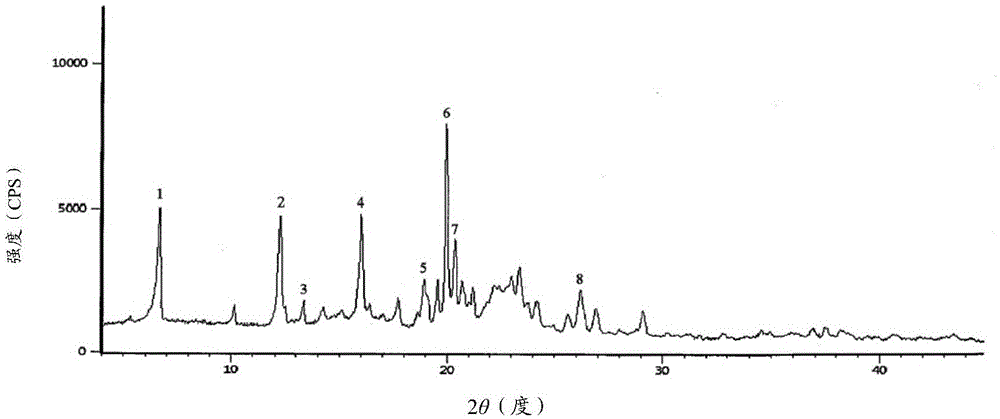
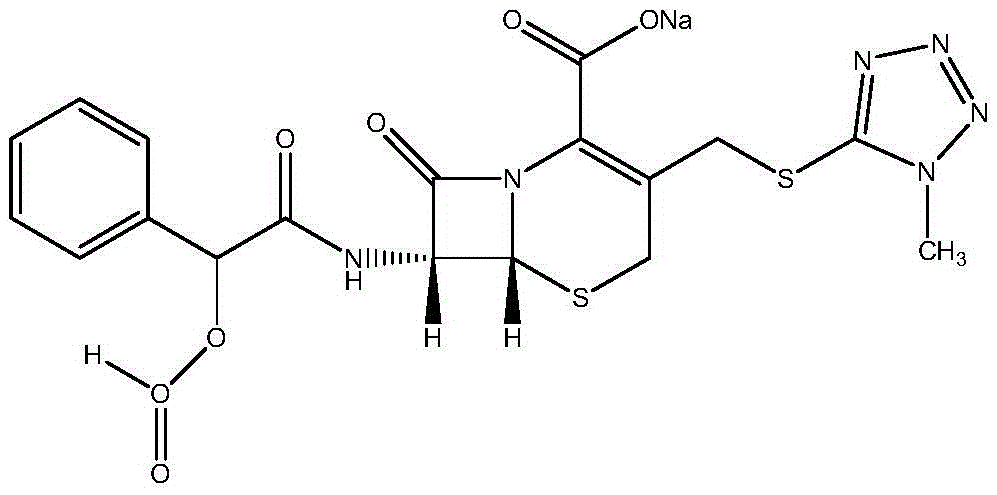
New crystal form cefamandole nafate compound prepared by adopting particle process crystal product molecular assembling and morphology optimizing technology and preparation
A technology of cefamandole sodium and its compound, which is applied in the field of medicine and can solve problems such as poor color, low content, and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Preparation of Cefamandole Sodium New Crystal Form Compound
[0028] (1) Add 10.5g of 7-ATCA and 100ml of dichloromethane to the reactor successively, and stir for 10min; continue to gradually add BSA15ml into the above solution; react at 25~30℃ for 3h, cool down to -5~-10℃; Add 8.1 g of formylmandelic acid chloride (dissolved in 25 ml of ethyl acetate). After the dropwise addition, the temperature is raised to 0-5° C. for 3 h. Add 100ml of deionized water at about 15°C and 100g of sodium acetate to the above reactor; stir for 30 minutes, let stand for 30 minutes to separate layers; separate the water layer, add 50ml of saturated sodium chloride solution to the organic layer, stir for 15 minutes, and combine the organic layers . Add 15 g of anhydrous magnesium sulfate to the organic layer, stir and dehydrate for 60 minutes; then add 2 g of activated carbon, stir and decolorize for 30 minutes; filter, and vacuum-dry the filtrate at 28° C. to obtain a solid...
Embodiment 2
[0037] Embodiment 2: Preparation of Cefamandole Sodium New Crystal Form Compound
[0038] (1) Add 11.4g of 7-ATCA and 100ml of dichloromethane to the reactor successively, and stir for 10min; continue to gradually add BSA15ml into the above solution; Add 11.4g of formylmandelic acid chloride (dissolved in 35ml of ethyl acetate), and after the dropwise addition, raise the temperature to 0-5°C and react for 3h. Add 100ml of deionized water at about 15°C and 100g of sodium acetate to the above reactor; stir for 30 minutes, let stand for 30 minutes to separate layers; separate the water layer, add 50ml of saturated sodium chloride solution to the organic layer, stir for 15 minutes, and combine the organic layers . Add 13.8g of anhydrous magnesium sulfate to the organic layer, stir and dehydrate for 60min; then add 2g of activated carbon, stir for 30min to decolorize; filter, and vacuum-dry the filtrate at 30°C to obtain a solid-liquid mixture of cefamandroic acid. .
[0039] (2...
Embodiment 3
[0042] Embodiment 3: Preparation of new crystal form compound of cefamandole sodium
[0043] (1) Add 11.4g of 7-ATCA and 100ml of dichloromethane to the reactor successively, and stir for 10min; continue to gradually add BSA15ml into the above solution; Add 20.5g of formylmandelic acid chloride (dissolved in 60ml of ethyl acetate), and after the dropwise addition, raise the temperature to 0-5°C and react for 3h. Add 200ml of deionized water at about 15°C and 200g of sodium acetate to the above reactor; stir for 30 minutes, let stand for 30 minutes to separate layers; separate the water layer, add 50ml of saturated sodium chloride solution to the organic layer, stir for 15 minutes, and combine the organic layers . Add 20.4g of anhydrous magnesium sulfate to the organic layer, stir and dehydrate for 60min; then add 2g of activated carbon, stir for 30min to decolorize; filter, and vacuum-dry the filtrate at 30°C to obtain a solid-liquid mixture of cefamandroic acid. .
[0044]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com