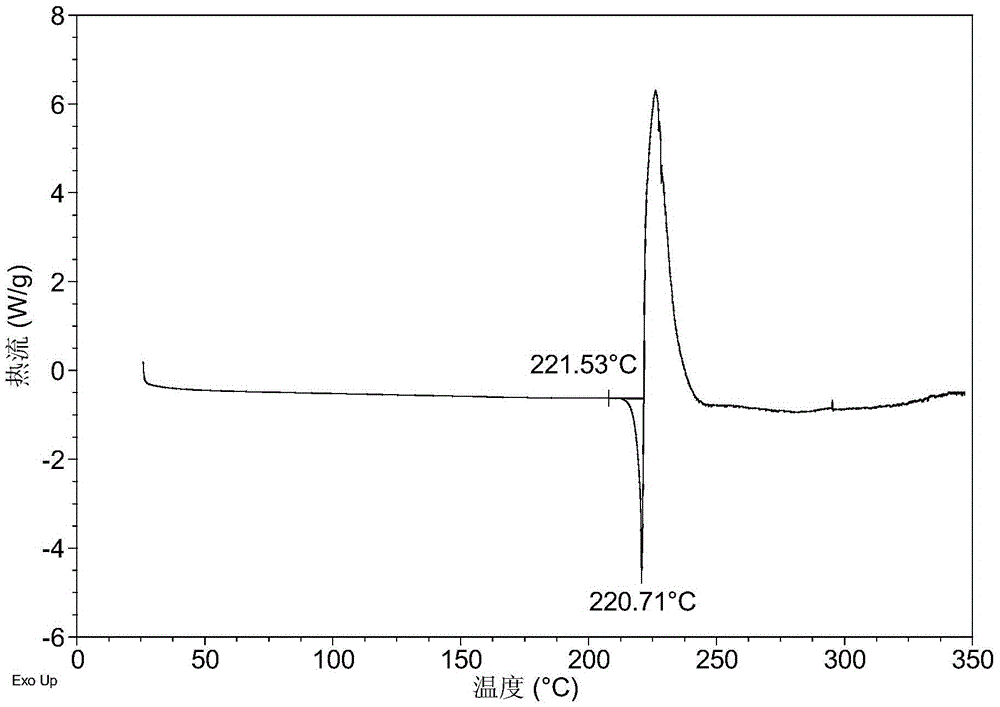
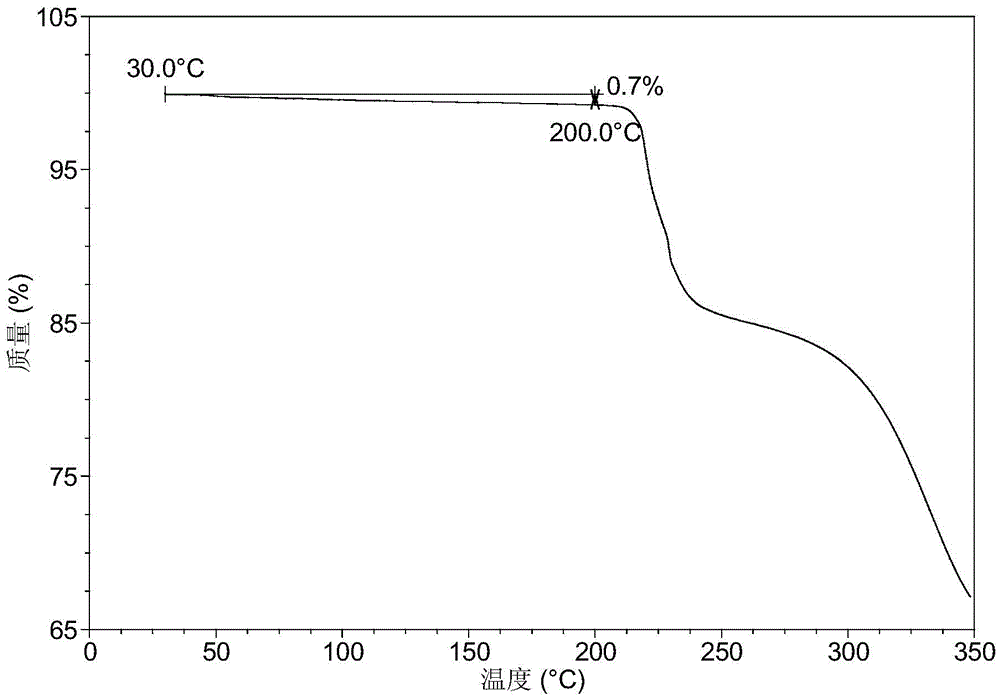
Crystal forms of orally-taken mitogen-activated protein kinase inhibitor and preparation method of crystal forms
A crystal form and solvent technology, applied in organic chemistry methods, organic chemistry, pharmaceutical formulations, etc., can solve problems affecting drug absorption and bioavailability, differences in clinical drug efficacy, solubility and stability, and achieve process purification effects Significant, convenient for long-term storage, and the effect of storage conditions is not harsh
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The preparation method of formula (I) compound crystal form A:
[0070] Dissolve 10.2 mg of the compound of formula (I) in 1.8 mL of methanol, filter through a 0.45 μm nylon filter head, and place the clear solution in a 1.5 mL vial, and volatilize at room temperature to obtain solid crystal Form A.
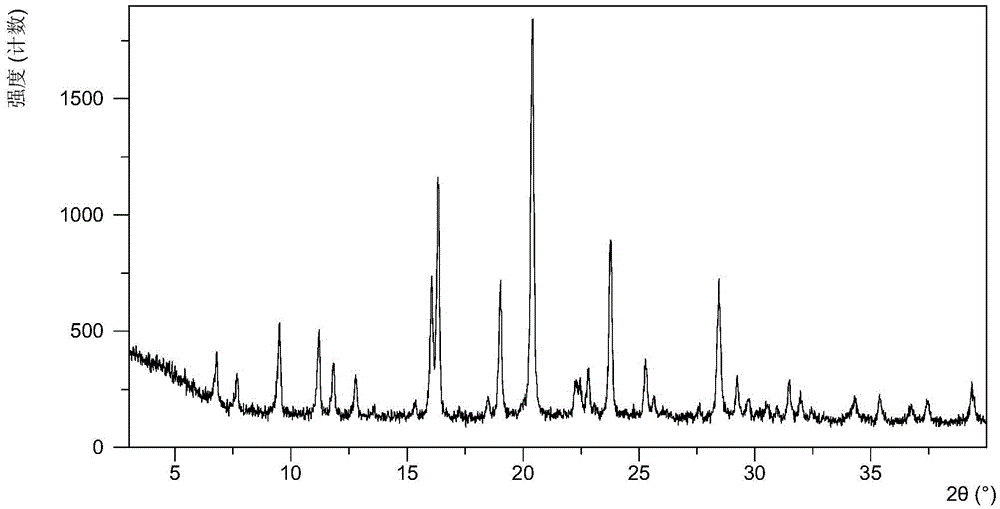
[0071] The X-ray powder diffraction data of the crystal form obtained in this embodiment are shown in Table 1, and its XRPD figure is as follows figure 1 .
[0072] Table 1
[0073]
[0074]
Embodiment 2
[0076] The preparation method of formula (I) compound crystal form A:
[0077] Add 12.0 mg of the compound of formula (I) into 1.2 mL of tetrahydrofuran, stir at room temperature at a rate of 500 r / min for three days, and centrifuge to obtain a solid which is Form A.
[0078] The X-ray powder diffraction data of the crystal forms obtained in this example are shown in Table 2.
[0079] Table 2
[0080]
[0081]
Embodiment 3
[0083] The preparation method of formula (I) compound crystal form A:
[0084] Dissolve 17.0 mg of the compound of formula (I) in 0.1 mL of dimethylformamide, then slowly add 0.2 mL of water dropwise while stirring, continue stirring for two days, and centrifuge to obtain a solid that is Form A.
[0085] The X-ray powder diffraction data of the crystal forms obtained in this example are shown in Table 3.
[0086] table 3
[0087]
[0088]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com