Docetaxel-oleic acid prodrug as well as nanostructure lipid carrier and application thereof
A technology of nano-structured lipids and nano-lipid carriers, which is applied in the application of oral drug delivery. In the field of nano-structured lipid carriers, it can solve the problems of low drug loading and limit clinical applications, and achieve good physical stability and stability. Good sex, improve the effect of oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of docetaxel-oleic acid prodrug.
[0033] Oleic acid (OA) was dissolved in a small amount of dichloromethane, under the catalysis of dicyclohexylcarboimide (DCC) and 4-dimethylaminopyridine (DMAP), N 2 Under the protection of ice bath for 2h, then with docetaxel at 25℃N 2 The reaction was carried out for 24 hours under protection, and the white powdery prodrug was obtained by separation and purification. The reaction formula is as follows:
[0034]
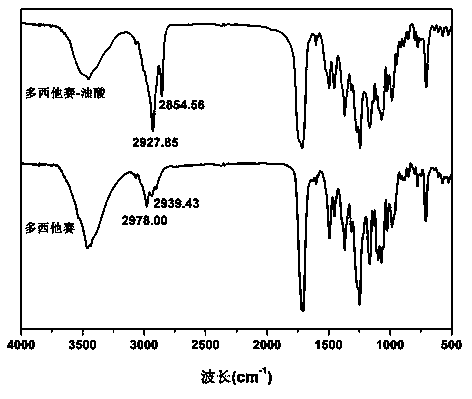
[0035] Determination by NMR 1 H-NMR hydrogen spectrum is determined the structure of prodrug in embodiment 1, and the solvent of selection is CDCl 3 , the result is as figure 1 . 1.158ppm, 1.783ppm, 5.229ppm, 8.131ppm are docetaxel H 7’–9’ ,H 14 ,H 20 ,H 25,29 characteristic peaks. 0.886ppm, 1.271ppm, 1.290ppm, 1.334ppm, 1.657ppm, 2.041ppm, 2.372ppm, 5.368ppm are oleic acid-CH 3 , CH 3 - (CH 2 ) 5 -CH 2 -, CH 3 -(CH 2 ) 5 - CH 2 -, -CH 2 - (CH 2 ) 3 -CH 2 -, - CH 2 -CH 2 CH 2...
Embodiment 2
[0039] Stability of docetaxel-oleic acid prodrug
[0040] Docetaxel-oleic acid prodrug and docetaxel were added to rat plasma respectively, placed in a shaker at 37°C, and the drug was measured at 0h, 2h, 4h, 6h, 8h, 12h, 24h, 48h, 72h, 96h, 120h content changes. The result is as Figure 4 shown. In rat plasma, the docetaxel-oleic acid prodrug was more stable than docetaxel.
Embodiment 3
[0042] Preparation of nanostructured lipid carriers based on core-match mechanism by emulsification ultrasonic method
[0043] The preparation process is as follows: a certain amount of glyceryl monostearate (6.7mg), oleic acid (3.3mg), docetaxel-oleic acid prodrug (8mg), PLV 2000 (3mg), dissolved in an appropriate amount of ethanol, heated to 80°C, and dissolved evenly under magnetic stirring. Will F 68 (10mg) was dissolved in 10mL water, heated to 80°C, and dissolved evenly under magnetic stirring. The above ethanol solution was quickly added to the aqueous solution, and magnetic stirring was maintained for 5 min in a water bath at 80°C. The above solution was ultrasonically treated (200W, 5min), and rapidly cooled in an ice bath to obtain a light blue opalescent liquid preparation. The docetaxel-oleic acid prodrug in the above method is replaced by docetaxel, coumarin-6 and DiR respectively, and other conditions remain unchanged, and the preparation contains docetaxel, c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com