Polypeptide capable of inhibiting matrix metalloproteinase 10 and application of polypeptide
A matrix metal, peptide inhibition technology, applied in the direction of peptide/protein components, peptides, enzymes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Chemical Synthesis of Peptides
[0013] The peptides were synthesized using Fmoc chemistry. The synthesis reaction is carried out from the C-terminus to the N-terminus. There are free amino groups on the Rink medium (available from AdvancedChemTech), and the amino acids are connected in the order from the C-terminus to the N-terminus. During each ligation step, the amino acid residues are activated, and the activation mixture contains 4 times as many HBTU, HOBt, DIEA and Fmoc-amino acids as there are free amino groups on the medium. After each amino acid attachment reaction, a pyridine / acetic acid / N-methylimidazole mixture was used to block the unattached free amino groups. After each amino acid linking reaction and before the next amino acid linking, the Fmoc-group on the medium should be removed, and the Fmoc-group should be removed using dimethylformamide containing 20% piperidine. Finally, after all amino acid residues are linked sequentially, the peptide is cle...
Embodiment 2
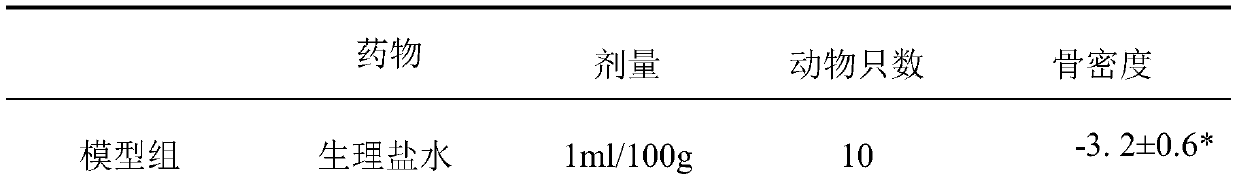
[0016] The IC50 value of the inhibitory polypeptide against matrix metalloproteinase 10 in vitro.
[0017] The enzyme activity of recombinant human matrix metalloproteinase 10 is detected by cleaving the fluorescently produced polypeptide substrate Mca-Arg-Pro-Lys-Pro-Val-Glu-Nval-Trp-Arg-Lys(Dnp)-NH 2 And detect the generated fluorescence value (excitation wavelength=328nm, detection wavelength=392nm). All assays were performed in 100 μl reactions at 37°C. The concentration of matrix metalloproteinase 10 during activation was 100 ng / μl (1 μM). At the beginning of the reaction, 10 μl of matrix metalloproteinase-10 storage solution (1 ng / μl) was added to the reaction system, and the final concentration of the substrate was 10 μM. The IC50 value of the matrix metalloproteinase 10 inhibitory polypeptide of Example 1 was 11.81 μmol.
Embodiment 3
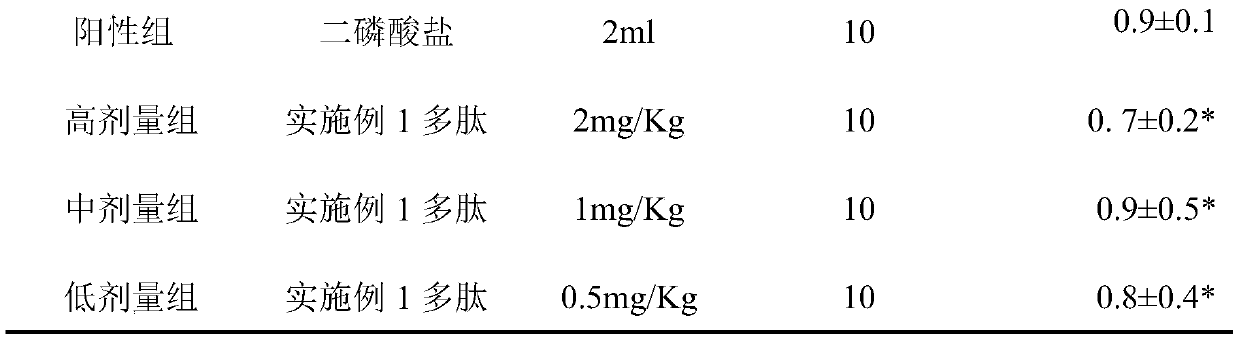
[0019] Inhibiting the EC50 of polypeptides on the growth of human osteoblasts in vitro.
[0020] The MTT colorimetric method was used. Human osteoblasts growing logarithmically, at 1.0×10 5 Add it into a 96-well culture plate and culture it for 24 hours. Add different concentrations of the experimental drug Example 1 polypeptide (Shanghai Jier Synthetic) to the experimental wells; add the same volume of solvent to the blank group. Set up five duplicate wells in each hole, after culturing for 48 hours, add MTT to each hole, after acting for 4 hours, add DMSO, incubate for 30 minutes, measure absorbance A value at 620nm place of microplate reader, according to the formula osteoblast proliferation rate=(1-experiment Absorbance value of group / absorbance value of control group)×100%. The calculated EC50 of the polypeptide of Example 1 was 4.31 μM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com