Diamine monomer with triphenylamine structure containing p-substituted cyclic amine, preparation method and application of diamine monomer
A technology of diamine monomers and cyclic amines, which is applied in the field of preparing polyamides with electrochromic properties, can solve problems such as instability of triphenylamine cations and coupling reactions, and achieve improved solubility, improved stability, and weakened The effect of interaction force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
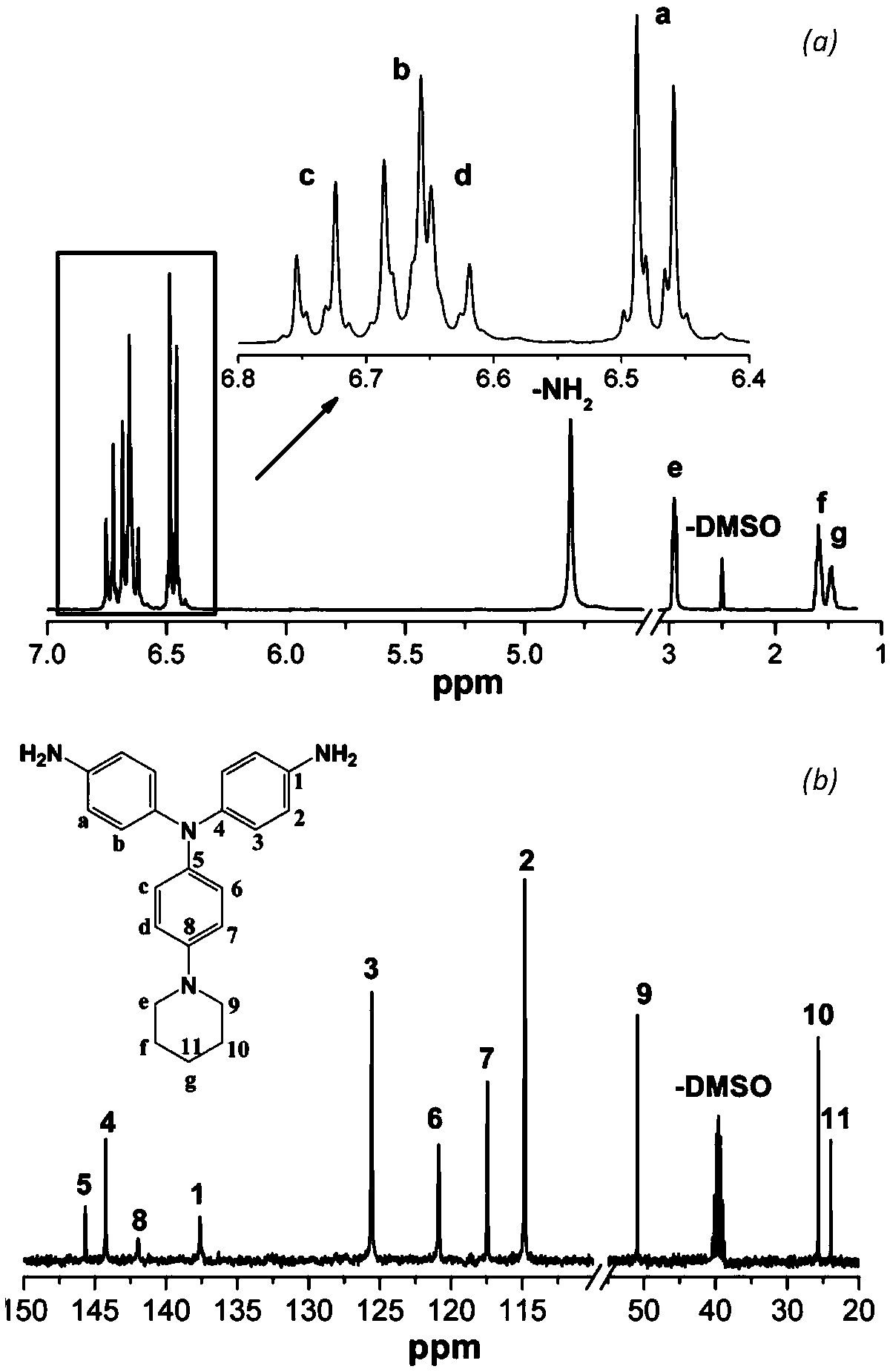
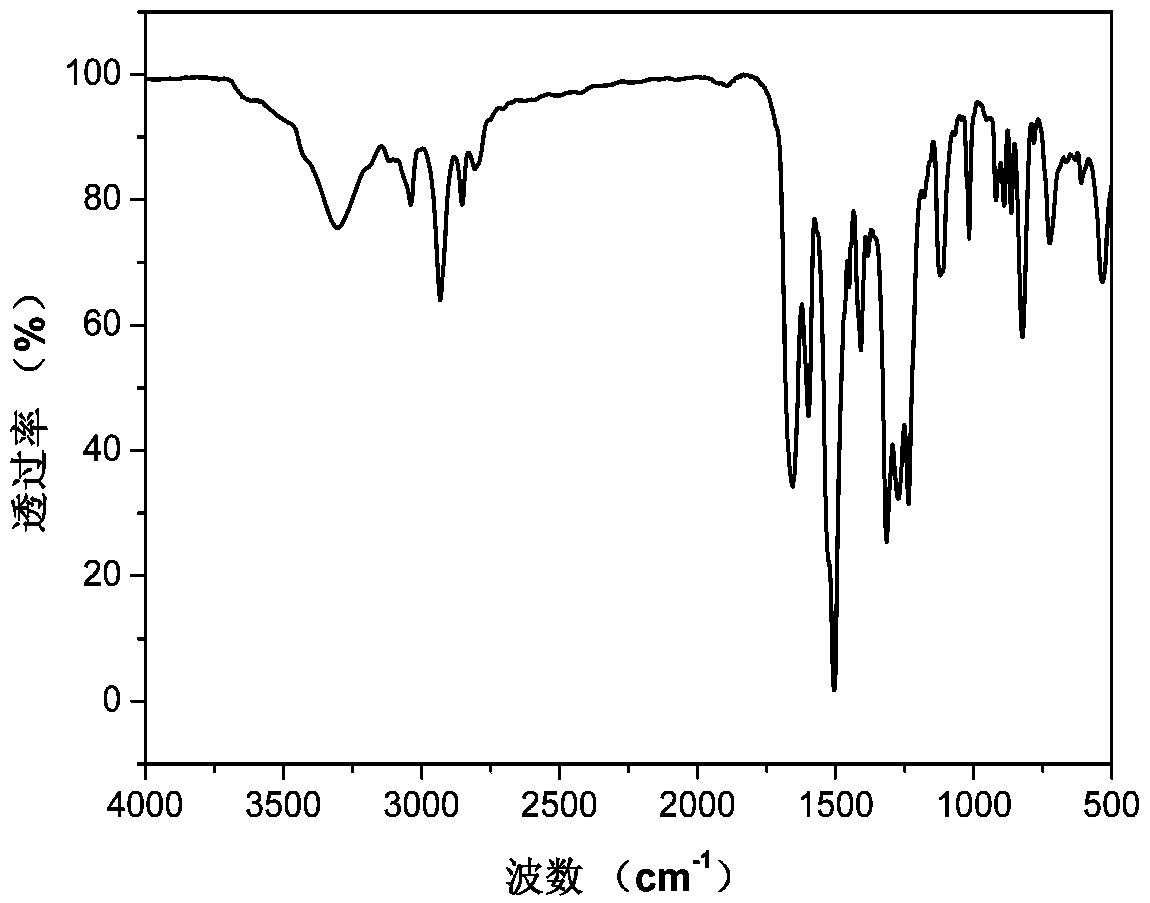
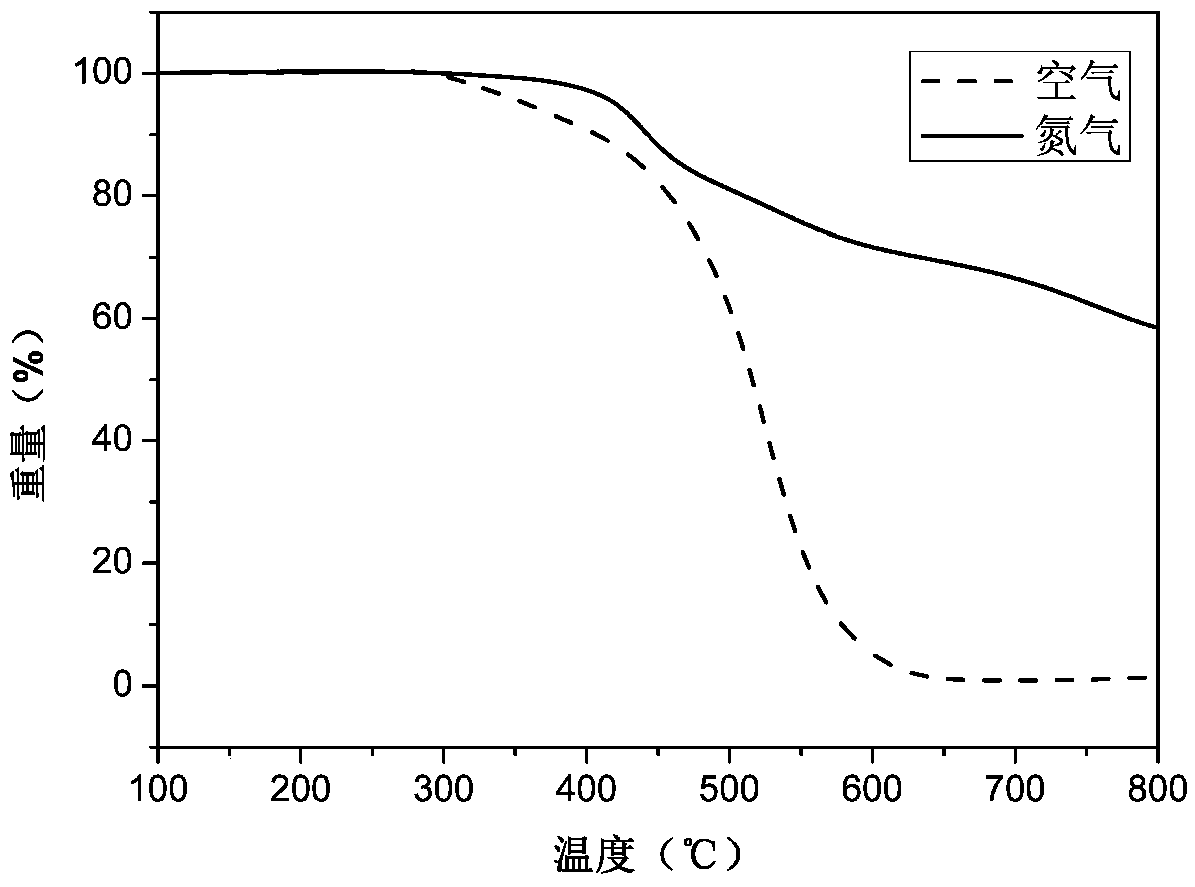
[0040] Example 1: Preparation of N,N-bis(4-aminophenyl)-4-piperidine aniline
[0041] The first step reaction: add 15.0g (176mmol) piperidine, 24.9g (176mmol) p-fluoronitrobenzene, 24.5g (176mmol) potassium carbonate in a 500mL three-necked flask equipped with mechanical stirring, add 250mL of N, N -Dimethylformamide was used as a solvent, stirred and reacted at 110°C for 5 hours under the protection of nitrogen. After cooling, the material was discharged in ice water, and the crude product was washed with water for 3 times. After drying, ethanol recrystallized to obtain yellow 4- Nitrophenyl piperidine powder 16.7g, productive rate is 46%;
[0042] The second step reaction: add 15.0 g of 4-nitrophenylpiperidine powder prepared in the first step reaction and 2.0 g of Pd with a mass fraction of 10% to a 500 mL three-necked flask equipped with a magnetic stirrer, a thermometer and a condenser tube. / C, add 180mL ethanol as a solvent, stir to obtain a suspension. After heating ...
Embodiment 2
[0047] Example 2: Preparation of N,N-bis(4-aminophenyl)-4-tetrahydropyrrole aniline
[0048] The first step reaction: add 10.0g (141mmol) tetrahydropyrrole, 21.9g (155mmol) p-fluoronitrobenzene, 21.5g (155mmol) potassium carbonate in a 250mL three-necked flask equipped with mechanical stirring, add 100mL of N, N-dimethylformamide was used as a solvent, stirred and reacted at 100°C for 8 hours under the protection of nitrogen. After cooling, the material was discharged in ice water. The crude product was washed 3 times with water, dried, and recrystallized from ethanol to obtain yellow 4 - 13.4g of nitrobenzene tetrahydropyrrole powder, the productive rate is 49%;
[0049] Second-step reaction: Add 10.0 g of 4-nitrobenzenetetrahydropyrrole powder prepared in the first step reaction, 1.5 g of 10% of Pd / C, add 150mL ethanol as a solvent, stir evenly to obtain a suspension. After that, it was heated to reflux, and 32.0 g of hydrazine hydrate with a mass fraction of 80% was slowl...
Embodiment 3
[0053] Example 3: Preparation of N,N-bis(4-aminophenyl)-4-(4-methylpiperidine)aniline
[0054] The first step reaction: add 10.0g (101mmol) 4-methylpiperidine, 14.9g (106mmol) p-fluoronitrobenzene, 14.6g (106mmol) potassium carbonate in a 250mL three-necked flask equipped with mechanical stirring, add 100mL N,N-dimethylformamide was used as a solvent, under stirring and nitrogen protection, reacted at 120°C for 12h, after cooling, the material was discharged in ice water, the crude product was washed 3 times with water, after drying, recrystallized from ethanol to obtain Yellow 4-nitrophenyltetrahydropyrrole powder 11.3g, yield rate is 51%;
[0055] The second step reaction: add 10.0 g of 4-nitrobenzene-4-methylpiperidine powder prepared in the first step reaction, 1.5 g mass fraction For 10% Pd / C, add 150mL ethanol as a solvent, stir evenly to obtain a suspension. After that, it was heated to reflux, and 30.0 g of hydrazine hydrate solution with a mass fraction of 80% was s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com