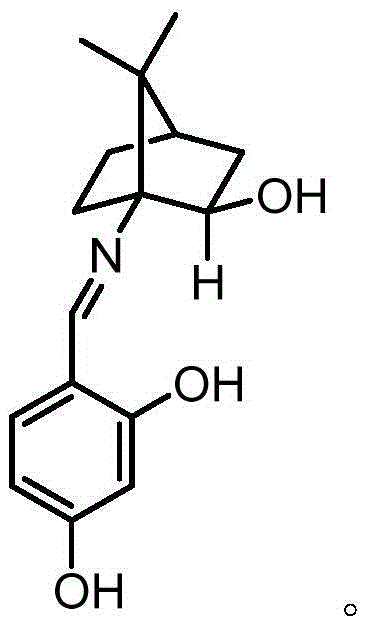
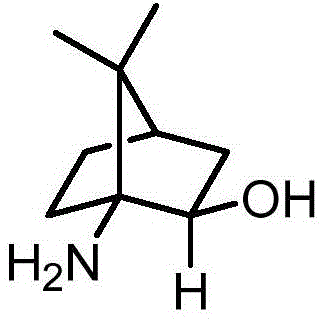
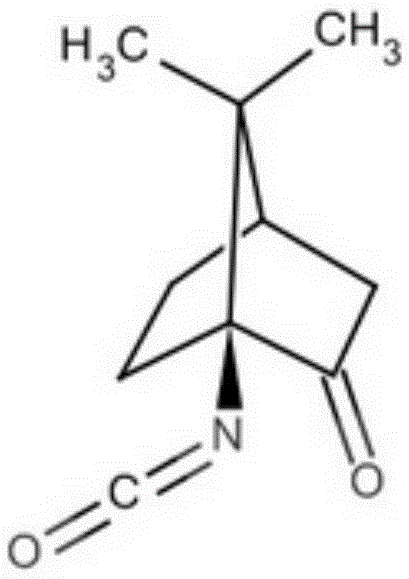
Camphor Schiff base, and preparation method and application thereof
A technology of Schiff base and camphor, which is applied in the field of asymmetric synthesis of β-nitro alcohols, can solve the problems of no research reports and achieve high catalytic efficiency, high yield, and high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. Dissolve 5.01g (28mmol) of 1-isocyanate-2-camphorone in 100mL of absolute anhydrous methanol, cool to 0°C, add 2.6g (7mmol) of cerium trichloride heptahydrate, after the addition, the reaction solution is cooled to -78°C, add 5.3g (140mmol) sodium borohydride in batches within 1 hour, and continue to stir at -78°C for 1 hour, then the reaction solution is warmed to -40°C, and continues to stir at this temperature for 2 hours , then the temperature of the reaction solution was slowly raised to room temperature, 50mL of 6mol / L KOH aqueous solution was added to the residual solution, and heated to reflux for 3 hours, the reaction solution was cooled to room temperature, extracted with dichloromethane (3 × 100mL), and the organic phases were combined, Dry over anhydrous sodium sulfate, filter, and remove dichloromethane from the filtrate by a rotary evaporator to obtain a white camphor amino alcohol crude product, which is further separated by column chromatography (silic...
Embodiment 2
[0026] Camphor Schiff base of the present invention is applied in the asymmetric Henry reaction of cuprous chloride catalyzed 4-nitrobenzaldehyde and nitromethane, and concrete usage method is as follows:
[0027] Under nitrogen protection, add 0.014g (0.05mmol) camphor Schiff base, 0.005g (0.05mmol) cuprous chloride and 2mL tert-butanol to the reaction flask, stir at room temperature for 2 hours, then add 0.54mL (20mmol ) nitromethane, and stirred for 30 minutes, then added 0.76g (0.5mmol) 4-nitrobenzaldehyde, after the TLC detection reaction was complete, removed low boiling point components with a rotary evaporator, and the crude product was separated by column chromatography to obtain ( R)-1-(4-nitrophenyl)-2-nitroethanol, the yield is 90%, and the ee value is 84%.
Embodiment 3
[0029] Camphor Schiff base of the present invention is applied in the asymmetric Henry reaction of cuprous chloride catalyzed 3-nitrobenzaldehyde and nitromethane, and concrete usage method is as follows:
[0030] Under nitrogen protection, add 0.014g (0.05mmol) camphor Schiff base, 0.005g (0.05mmol) cuprous chloride and 2mL tert-butanol to the reaction flask, stir at room temperature for 2 hours, then add 0.54mL (20mmol ) nitromethane, and stirred for 30 minutes, then added 0.76g (0.5mmol) 3-nitrobenzaldehyde, after the TLC detection reaction was complete, removed low boiling point components with a rotary evaporator, and the crude product was separated by column chromatography to obtain ( R)-1-(3-nitrophenyl)-2-nitroethanol, the yield was 82%, and the ee value was 80%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com