Apremilast crystal form, and preparation method, pharmaceutical composition and application thereof
A technology of composition and crystal form, applied in the field of medicinal chemical crystallization, to reduce the risk of curative effect decline and safety risk, good application effect, and reduce toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0059] Apremilast was synthesized according to the synthesis method of Patent Document WO2009120167A1 Example 2, specifically: (S)-2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulfonyl)-ethyl -N-acetyl-L-leucine salt of 2-yl-amine (25g, 56mmol), 3-acetylaminophthalic anhydride (12.1g, 58.8mmol) and glacial acetic acid (250mL) were mixed and refluxed overnight, Then the mixture was lowered to less than 50°C, and the solvent was removed by vacuum drying. The obtained solid was dissolved in ethyl acetate, and the obtained solutions were respectively water (250mL×2), saturated aqueous sodium bicarbonate (250mL×2) and saturated aqueous sodium chloride (250mL ×2) Rinse. After the solution was dried with anhydrous sodium sulfate, the solvent was evaporated in vacuo, and the resulting solid was stirred in a mixed solvent of ethanol (150 mL) and acetone (75 mL) for 3 hours to recrystallize, filtered with suction, and the filter cake was rinsed with ethanol (100 mL×2). The obtained solid was ...
preparation example 2
[0062]Apremilast crystal form B was prepared according to the preparation method in Example 5.12.3 of patent document WO2009120167A1, specifically: 2 g of apremilast was dissolved in 200 mL of methanol at 50 ° C, cooled naturally in a water bath and stirred to room temperature, and the solid Filtration and vacuum drying at 45°C for 16 hours gave Apremilast Form B.
[0063] XRPD image as shown figure 1 Shown, and the XRPD of apremilast crystal form B disclosed in patent document WO2009120167 Figure 1 To;
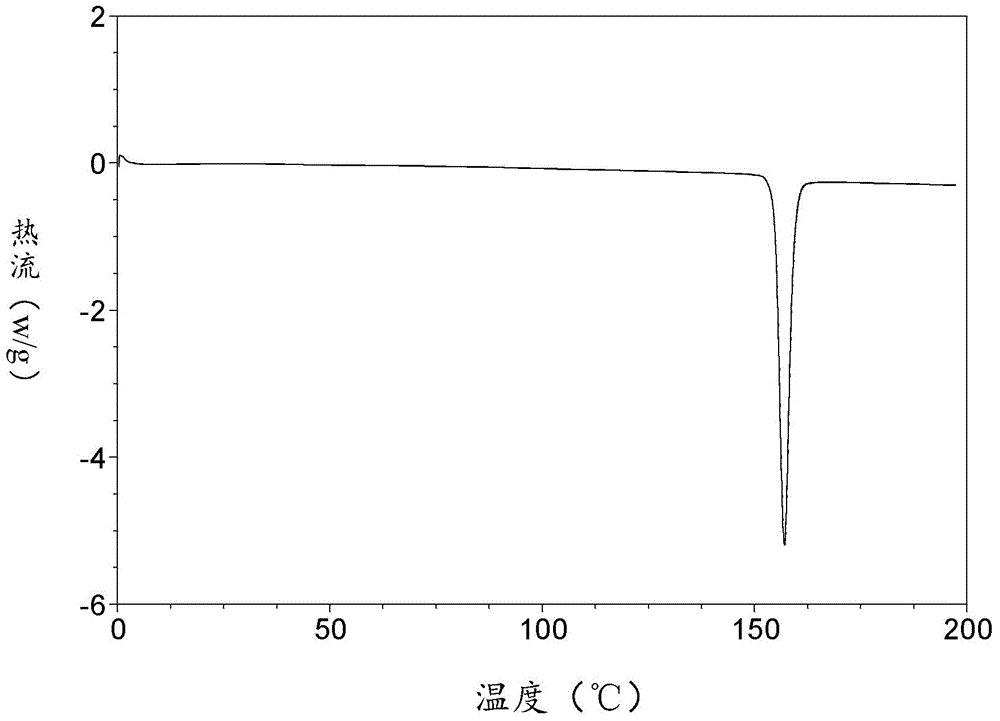
[0064] DSC diagram as shown figure 2 As shown, it shows that the melting point of the sample is 155-157°C;
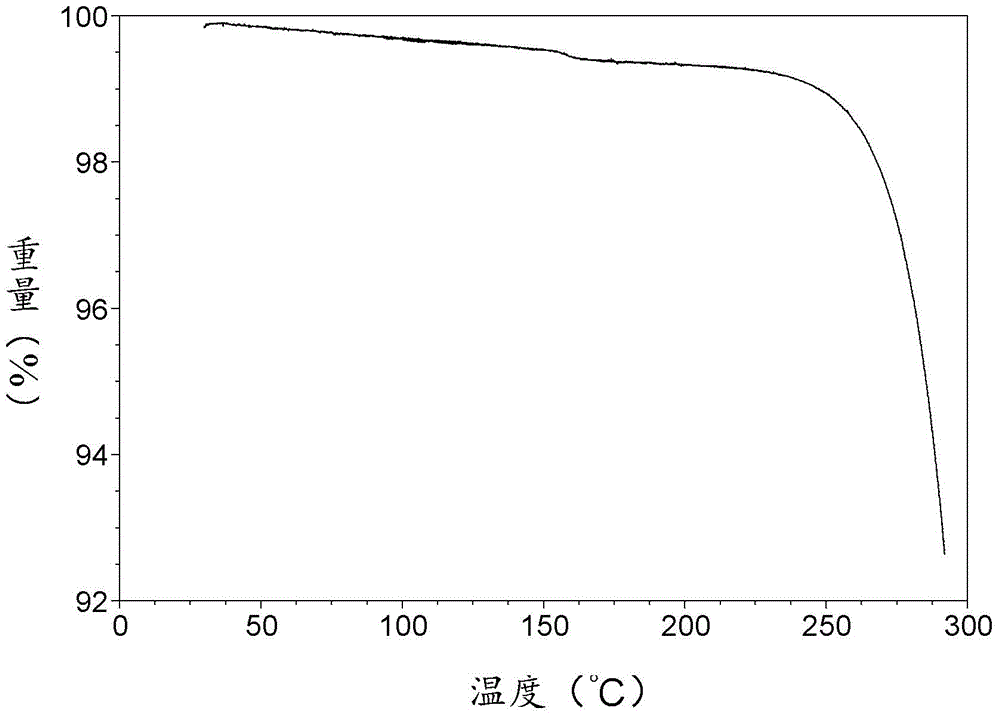
[0065] TGA figure such as image 3 As shown, it shows that the sample is anhydrous, and the decomposition temperature is 252°C.
Embodiment 1
[0067] Weigh 50 mg of Apremilast crystal form B prepared in Preparation Example 2, dissolve it in 1 mL of methyl ethyl ketone at room temperature, then add 20 μL of water to form a solution, and volatilize the glass vial containing the obtained solution until the solution is dry, and the obtained solid Vacuum drying at 30°C for 24 hours gave apremilast crystal form I with a yield of 46 mg and a molar yield of 90.2%.
[0068] XRPD image as shown Figure 4 shown;
[0069] DSC diagram as shown Figure 5 As shown, it shows that the sample contains a dehydration endothermic peak and a melting endothermic peak at 129-174 ° C, wherein the dehydration temperature is 161 ± 2 ° C (peak value), and the melting point is 166 ± 2 ° C (peak value).
[0070] TGA figure such as Image 6 As shown, it shows that the sample has a weight loss of about 2.4% between 140°C and 166°C, which means that each mole of apremilast crystal form I contains 0.5 moles of water, which is a hemihydrate; the dec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com