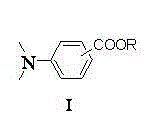Preparation method of N,N-dimethyl benzoate composite
A technology of dimethylaminobenzoic acid and ester compounds, which is applied in the field of preparing N from esters and alcohols, can solve the problems of less preparation methods, unfriendly environment, and more waste water, achieve mild and easy-to-control reaction conditions, and reduce production The effect of cost and raw material is cheap and easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
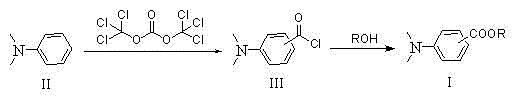
[0044] Embodiment 1: the preparation of p-dimethylaminobenzoyl chloride
[0045] In a 500ml four-neck flask, add N,N-dimethylaniline (72.7g, 0.6mol) and 30ml of chlorobenzene, stir evenly, and dissolve bis(trichloromethyl)carbonate (29.7g, 0.1mol) In 60ml of chlorobenzene, slowly add dropwise into the chlorobenzene solution of N,N-dimethylaniline, after 2 hours, slowly raise the temperature to 65°C, keep warm for reaction, monitor the reaction by TLC or GC, after the reaction is complete, drop to At room temperature, chlorobenzene was recovered by distillation at normal pressure and then under reduced pressure. After the recovery of chlorobenzene, the excess raw material N,N-dimethylaniline was recovered, and finally 33.1 g of the product was obtained by rectification under reduced pressure (conditions for collecting fractions: 175~180°C / 15mmHg), yield 60%, purity 99.0%.
Embodiment 2
[0046] Embodiment 2: the preparation of p-dimethylaminobenzoyl chloride
[0047] In a 500ml four-neck flask, add N,N-dimethylaniline (121.2g, 1.0mol) and 60ml of chlorobenzene, stir evenly, and dissolve bis(trichloromethyl)carbonate (29.7g, 0.1mol) In 120ml of chlorobenzene, slowly add dropwise to the chlorobenzene solution of N,N-dimethylaniline, after 2 hours, slowly raise the temperature to 65°C, keep warm for reaction, monitor the reaction by TLC or GC, after the reaction is complete, drop to At room temperature, chlorobenzene was recovered by distillation at normal pressure and then under reduced pressure. After the recovery of chlorobenzene, the excess raw material N,N-dimethylaniline was recovered, and finally 44.1 g of the product was obtained by rectification under reduced pressure (conditions for collecting fractions: 175~180°C / 15mmHg), yield 80%, purity 99.1%.
Embodiment 3
[0048] Embodiment 3: the preparation of o-dimethylaminobenzoyl chloride
[0049] In a 500ml four-neck flask, add N,N-dimethylaniline (121.2g, 1.0mol) and 60ml of chlorobenzene, stir evenly, and dissolve bis(trichloromethyl)carbonate (29.7g, 0.1mol) In 120ml of chlorobenzene, slowly add dropwise to the chlorobenzene solution of N,N-dimethylaniline, after 2 hours, slowly raise the temperature to 40°C, keep warm for reaction, monitor the reaction by TLC or GC, after the reaction is complete, use gas chromatography It shows that the ratio of o-dimethylaminobenzoyl chloride: p-dimethylaminobenzoyl chloride in the reaction solution is 75:25. After recovering the solvent and excess raw materials, o-dimethylaminobenzoyl chloride can be purified by vacuum distillation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com