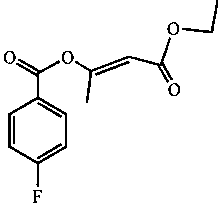
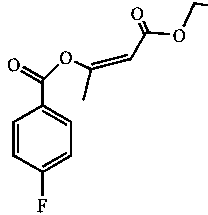
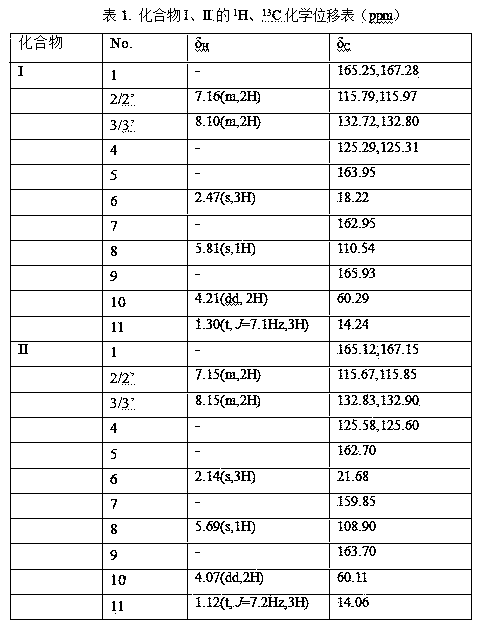
Enol ethyl acetoacetate E/Z type derivatives and synthesis and preparation method thereof
A technology of ethyl acetoacetate enol and ethyl acetoacetate, which is applied in the field of ethyl acetoacetate enol E/Z derivatives and their synthesis and preparation, and can solve problems such as affecting ability and affecting structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: 1.26 mL of ethyl acetoacetate, 2 mL of pyridine and 6 mL of chloroform were simultaneously added to a 50 mL round bottom flask, and a constant pressure dropping funnel with 1.18 mL of 4-fluorobenzoyl chloride was connected to the mouth of the flask. Fix the round bottom flask on a magnetic stirrer and turn on the stirring switch. At this time, the plunger of the dropping funnel was rotated, and 4-fluorobenzoyl chloride was slowly added dropwise within 1 hour for reaction, and the reaction was continued for 1 hour after the dropwise addition. After the reaction, the reaction solution was transferred to a separatory funnel and washed dozens of times with distilled water. Then use anhydrous Na 2 SO 4 After drying, use a vacuum rotary evaporator to evaporate excess chloroform in a water bath at 35°C. The obtained liquid was developed with silica gel TLC (n-hexane-chloroform-acetonitrile: 5:4:0.6), and the yellow-green band under the fluorescence was scraped of...
Embodiment 2
[0018] Example 2: 1.26 mL of ethyl acetoacetate, 4 mL of pyridine and 6 mL of chloroform were simultaneously added to a 50 mL round bottom flask, and a constant pressure dropping funnel with 1.18 mL of 4-fluorobenzoyl chloride was connected to the mouth of the flask. Fix the round bottom flask on a magnetic stirrer and turn on the stirring switch. At this time, the plunger of the dropping funnel was rotated, and the 4-fluorobenzoyl chloride was slowly added dropwise within 0.5 h for reaction, and the reaction was continued for 0.5 h after the dropwise addition. After the reaction, the reaction solution was transferred to a separatory funnel and washed dozens of times with distilled water. Then use anhydrous Na 2 SO 4 After drying, use a vacuum rotary evaporator to evaporate excess chloroform in a water bath at 35°C. The obtained liquid was developed with silica gel TLC (n-hexane-chloroform-acetonitrile: 5:4:0.6), and the yellow-green band under the fluorescence was scraped ...
Embodiment 3
[0019] Example 3: 1.26 mL of ethyl acetoacetate, 4 mL of pyridine and 6 mL of chloroform were simultaneously added to a 50 mL round bottom flask, and a constant pressure dropping funnel with 1.77 mL of 4-fluorobenzoyl chloride was connected to the mouth of the flask. Fix the round bottom flask on a magnetic stirrer and turn on the stirring switch. At this time, the plunger of the dropping funnel was rotated, and 4-fluorobenzoyl chloride was slowly added dropwise within 0.5 h for reaction, and the reaction was continued for 1 h after the dropwise addition. After the reaction, the reaction solution was transferred to a separatory funnel and washed dozens of times with distilled water. Then use anhydrous Na 2 SO 4 After drying, use a vacuum rotary evaporator to evaporate excess chloroform in a water bath at 35°C. The obtained liquid was developed with silica gel TLC (n-hexane-chloroform-acetonitrile: 5:4:0.6), and the yellow-green band under the fluorescence was scraped off th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com