Saccharose containing roflumilast tablets and preparation method thereof
A technology of roflumilast tablet and roflumilast, which is applied in the field of medicine and can solve the problems of sticking to the wall of the pulverizing machine, easily blocking the feeding port and the discharging port, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Mix roflumilast and sucrose powder passed through the 80-mesh sieve at a ratio of 1:40, 1:20:, 1:10, and 1:6, respectively, and add each mixture to the In the sugar outlet of the centrifugal spinning machine (cotton candy machine), the heating temperature is about 186°C. When the powder starts to melt, the centrifugal device is turned on, and the mixture of roflumilast and sucrose is thrown out at a speed of about 3500r / min. into filaments and collected with a glass rod to obtain roflumilast-sucrose filaments with a weight ratio of roflumilast to sucrose of 1:40, 1:20:, 1:10, and 1:6, respectively.
[0033]Weigh 5 mg of roflumilast through a 200-mesh sieve and roflumilast-sucrose silk containing the same amount of roflumilast, and place them in a 500ml beaker, and then add 500ml of distilled water to the beaker. Turn on the ultrasonic instrument, put the beaker with the raw material drug and the roflumilast-sucrose silk in the ultrasonic water bath, and ultrasonically f...
Embodiment 2
[0036] Prescription of Roflumilast Tablets Containing Roflumilast 0.5mg (1000 Tablets)
[0037]
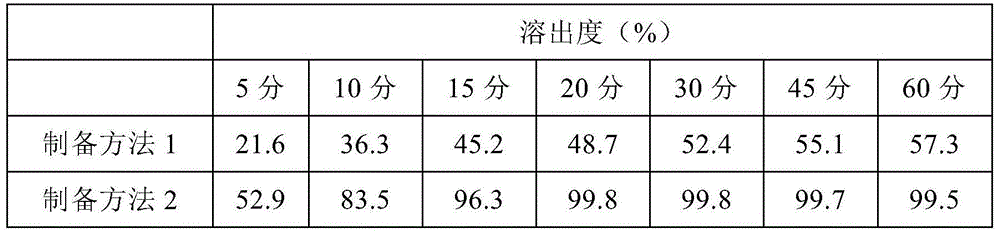
[0038] Preparation method 1: crush roflumilast and pass through a 200-mesh sieve, and crush sucrose and pass through a 80-mesh sieve. Mix the prescribed amount of roflumilast (200 mesh) with the prescribed amount of sucrose evenly, then add the prescribed amount of pregelatinized starch, direct-pressed mannitol and microcrystalline cellulose PH102, and then add the prescribed amount of stearic acid Magnesium, mixed evenly, press according to the theoretical tablet weight to obtain roflumilast tablets containing roflumilast 0.5mg with an average tablet weight of about 66.2mg.
[0039] Preparation method 2 Pass roflumilast through an 80-mesh sieve, crush sucrose and pass through an 80-mesh sieve. Mix roflumilast and sucrose through a 80-mesh sieve evenly in a weight ratio of 1:10, and add the mixture to the sugar discharger of the centrifugal spinning machine (cotton candy machi...
Embodiment 3
[0045] Prescription of Roflumilast Tablets Containing Roflumilast 0.5mg (1000 Tablets)
[0046]
[0047] Preparation method Pass roflumilast through 80-mesh sieve, crush sucrose and pass through 80-mesh sieve. Mix roflumilast and sucrose passing through a 80-mesh sieve evenly in a weight ratio of 1:30, and add the mixture to the sugar discharger of the centrifugal spinning machine (cotton candy machine) in a dry environment at 5°C, and the heating temperature is about At 186°C, when the powder starts to melt, turn on the centrifuge device, and at a speed of about 3500r / min, spin out the mixture of roflumilast and sucrose into silk and collect it with a glass rod. In a dry environment at 12°C, add the prescribed amount of corn starch to the prescribed amount of roflumilast-sucrose silk and mix, so that the roflumilast-sucrose silk is fully broken and mixed evenly with the corn starch. At room temperature, mix the above mixture with the prescribed amount of direct-compressed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com