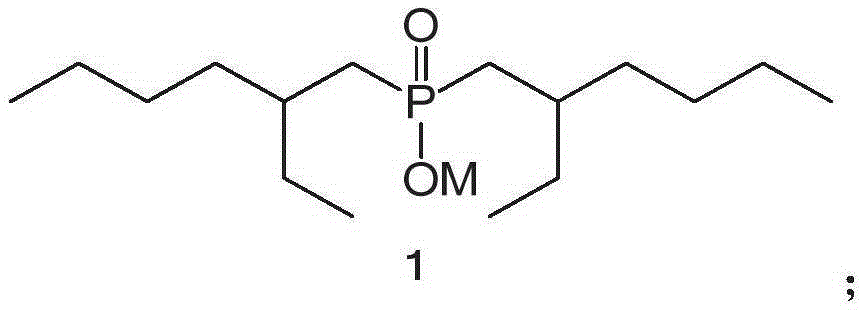
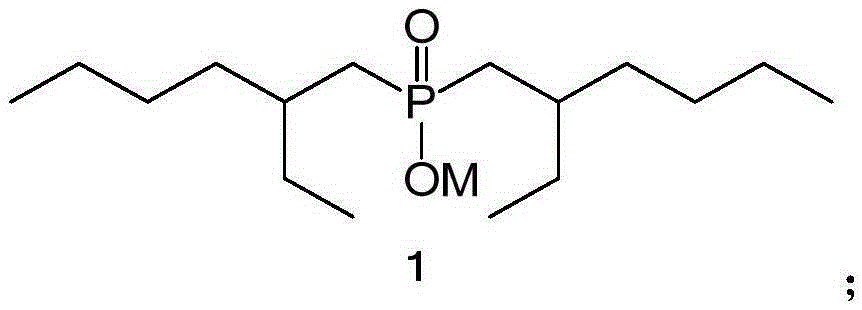
Method for removing iron through solvent extraction
A technology for extraction and removal of iron and solvent, applied in the field of solvent extraction and removal of iron, can solve the problems of large acid-base consumption, difficulty in stripping, large loss of valuable metals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] P227 extractant is dissolved in kerosene to prepare 0.5mol / L organic phase, and the aqueous phase contains Fe 3+ 1.03g / L, pH=2.1, in the case of unsaponified organic phase and water phase ratio of 1:4, the extraction temperature is 25 ℃, the time is 10min, the organic phase and the water phase are contacted twice to obtain 2.04 g / L loaded organic phase, the extraction rate is 99.03%; 3+ The organic phase was contacted twice at a temperature of 25°C, a time of 5 min, and a ratio (O / A) of 1:1, and the loaded Fe 3+ The organic phase extraction rate is 99.10%. Fe in the feed liquid under the experimental conditions 3+ Almost complete extraction, the stripping rate after extraction is also high, the organic phase can be recycled, and P227 extracts Fe 3+ It can be used in sulfuric acid system.
Embodiment 2
[0061] P227 extractant is dissolved in kerosene to prepare 0.5mol / L organic phase, and the aqueous phase contains Fe 3+ 5.17g / L, pH = 1.9, after the organic phase was saponified by potassium hydroxide 21%, the ratio of the organic phase to the aqueous phase was 1:1, the extraction temperature was 25°C, and the extraction time was 10 minutes. The organic phase was in contact with the aqueous phase for 1 Obtain the organic phase of 5.13g / L loading once, and extraction rate is 99.23%; 3+ The organic phase was contacted twice at a temperature of 25°C, a time of 5 min, and a ratio (O / A) of 1:1, and the loaded Fe 3+ The organic phase extraction rate is 99.17%. Fe in the feed liquid under the experimental conditions 3+ Almost complete extraction, the stripping rate after extraction is also high, and the organic phase can be recycled.
Embodiment 3
[0063] P227 extractant is dissolved in sulfonated kerosene to prepare 0.8mol / L organic phase, and the aqueous phase contains Fe 3+ 10.56g / L, pH = 2.2, the organic phase was saponified by sodium hydroxide 30% compared with the aqueous phase at a ratio of 1:1, the extraction temperature was 25°C, and the extraction time was 15 minutes. The organic phase was in contact with the aqueous phase for 1 Obtain the organic phase of 10.49g / L loading once, and extraction rate is 99.34%; 3+ The organic phase was contacted 3 times at a temperature of 25 °C for 5 min and a ratio (O / A) of 1:1 to load Fe 3+ The organic phase extraction rate is 99.31%. Fe in the feed liquid under the experimental conditions 3+ Almost complete extraction, the stripping rate after extraction is also high, the organic phase can be recycled, and P227 extracts Fe 3+ Can be used in hydrochloric acid system.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com