Enzalutamide intermediate preparation method
A technology of enzalutamide and intermediates, applied in the field of chemical synthesis, can solve the problems of expensive, limited application, and not very common sources of raw materials, and achieve the effect of low price, simple operation, and large-scale industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The molar ratio of 4-bromo-2-fluoro-N-methylbenzamide, 2-amino-isobutyric acid, catalyst, auxiliary catalyst, acid binding agent, and water is 1:1.5:0.1:0.1:4:2.3 .
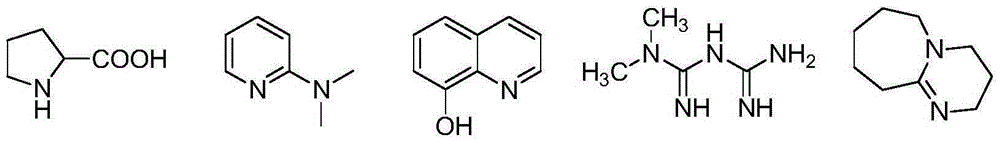
[0025] Add 4-bromo-2-fluoro-N-methylbenzamide (10g, 43.1mmol), 2-amino-isobutyric acid (6.7g, 64.7mmol), potassium carbonate (23.8g, 172.4mmol) in a single-necked flask ), proline (0.7g, 4.31mmol), water (1.8ml, 100mmol) were dissolved in DMF (60ml), replaced with nitrogen under stirring, then added CuCl (0.45g, 4.31mmol), heated to 100℃, reacted for 24h . After the completion of the reaction, first add water (120ml) to dilute the mixture, then add dichloromethane for extraction, remove the organic phase, adjust the pH to 4 for the aqueous phase with 1mol / L citric acid solution, and filter the precipitated solid. The solid was washed three times with a mixed solution of water and ethanol (100:1) to obtain 9.5 g of pure white solid. Yield 87%, product melting point: 210-211°C, HPLC purity 99.5%, HRMS[M+1] +...
Embodiment 2
[0029] The molar ratio of 4-bromo-2-fluoro-N-methylbenzamide, 2-amino-isobutyric acid, catalyst, auxiliary catalyst, acid binding agent, and water is 1:1:0.02:0.02:3:1.3 .
[0030] Add 4-bromo-2-fluoro-N-methylbenzamide (10g, 43.1mmol), 2-amino-isobutyric acid (4.7g, 43.1mmol), sodium carbonate (13.7, 129.3mmol) in a single-necked flask , N,N-Dimethyl-2-aminopyridine (0.105g, 0.862mmol), water (1ml, 55.6mmol) were dissolved in DMSO (60ml), replaced with nitrogen under stirring, and then added CuI (0.163g, 0.862mmol) , Heat to 110°C, react for 20h. After the reaction is completed, water (120ml) is added to dilute the mixture, and then ethyl acetate is added for extraction to remove the organic phase. The water phase was adjusted to pH=3 with 1 mol / L hydrochloric acid solution, and the precipitated solid was filtered; the solid was washed three times with a mixed solution of water and ethanol (100:1) to obtain 9.3 g of pure product. Yield 85%, product melting point: 210-212°C, H...
Embodiment 3
[0032] The molar ratio of 4-bromo-2-fluoro-N-methylbenzamide, 2-amino-isobutyric acid, catalyst, auxiliary catalyst, acid binding agent, and water is 1:2:0.25:0.25:6:2.6 .
[0033] Add 4-bromo-2-fluoro-N-methylbenzamide (10g, 43.1mmol), 2-amino-isobutyric acid (9.4g, 86.2mmol), triethylamine (26.1g, 258.6 mmol), 8-hydroxyquinoline (1.57g, 10.8mmol), and water (2ml, 111mmol) were dissolved in DMF (60ml), replaced with nitrogen under stirring, and then added CuBr (1.54g, 10.8mmol), heated to 120°C and refluxed , Reaction for 24h. After the reaction is completed, first add water (120ml) to dilute the mixture, and then add chloroform for extraction. The aqueous phase is adjusted to pH=5 with 1mol / L citric acid solution, and the precipitated solid is filtered; the solid is washed three times with a mixed solution of water and ethanol (100:1). The pure product is 9.1g. The yield is 83%, the product melting point: 209-210°C, the HPLC purity is 99.2%, HRMS[M+1] + :255.1076.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com