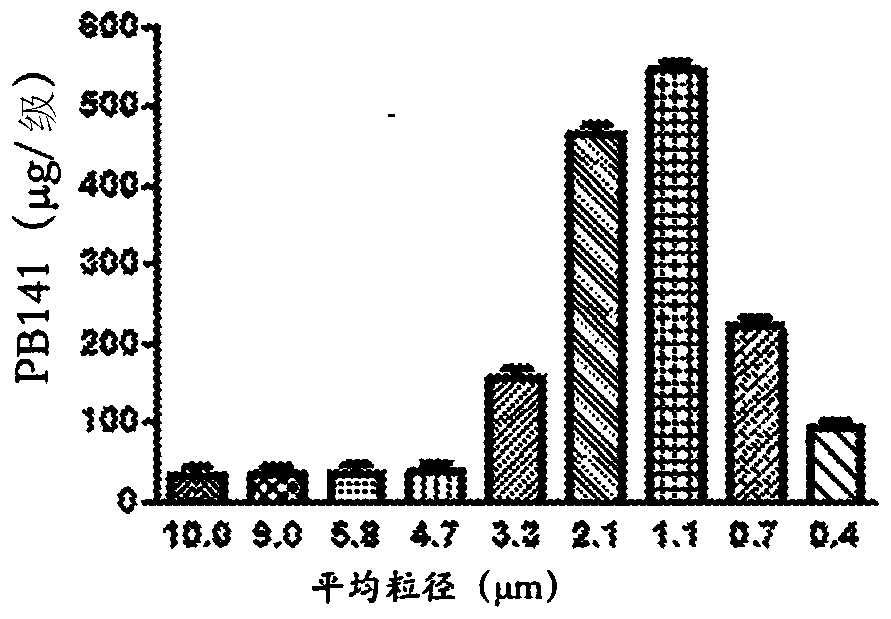
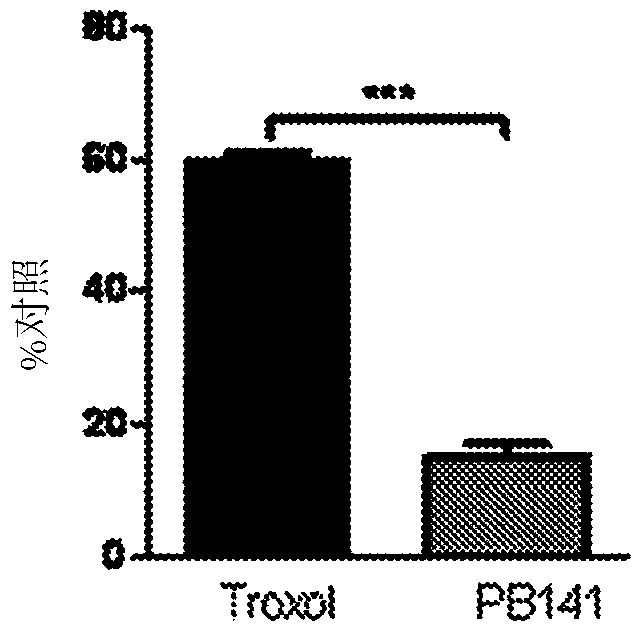
Treatment of lung and other conditions
A technology of alkyl compounds, applied in the field of treatment of lung diseases and other diseases, can solve the problems of invasion of normal tissues, cell proliferation, mutation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0698] General Synthesis of PB Compounds
[0699] A solution of pyrazine-2-carbaldehyde (4.62 mmol) in pure ethanol (1 ml) was added dropwise to NaOH (0.75 mmol) and ketone (acetone) in pure ethanol under slow stirring over 10 minutes at room temperature and H 2 o 2 (14ml; 1:1 ratio) of the mixture in solution. The solution will turn yellow followed by the formation of a yellow precipitate within 10 minutes. The reaction was stirred at room temperature for 6 h, then the yellow solid was removed by filtration and washed with water; the organic phase was then washed with anhydrous MgSO 4 Dried and concentrated under vacuum to give a pale yellow powder representing PB137 in 97% purity.
Embodiment 2
[0701] Synthesis of PB compounds with acetone
[0702] After 10 minutes at room temperature, under slow stirring, pyrazine-2-carbaldehyde [or 3-aminopyrazine-2-carbaldehyde; 6-amino-5-hydroxypyrazine-2-carbaldehyde; 5-hydroxy- 6-methoxypyrazine-2-carbaldehyde; 3-thiocyanatopyrazine-2-carbaldehyde; 5-(chloromethylthio)pyrazine-2-carbaldehyde; 5-acetylpyrazine-2-carbaldehyde; 6-(trifluoromethoxy)pyrazine-2-carbaldehyde; 5-fluoropyrazine-2-carbaldehyde; 5-(methylthio)pyrazine-2-carbaldehyde; 5-(chloromethylthio)pyrazine -2-carbaldehyde; 6-tert-butoxypyrazine-2-carbaldehyde; 5-acetylpyrazine-2-carbaldehyde; 6-trifluoromethoxypyrazine-2-carbaldehyde; 6-amino-5-hydroxypyridine oxazine-2-carbaldehyde; 5-hydroxy-6-methoxypyrazine-2-carbaldehyde; 6-(trifluoromethyl)pyrazine-2-carbaldehyde: (4.62mmol)] in pure ethanol (1ml) The solution was added dropwise to NaOH (0.75 mmol) and acetone in absolute ethanol and H 2 o 2 (14ml; 1:1 ratio) of the mixture in solution. The solution will ...
Embodiment 3
[0704] Synthesis of PB Compounds Using Cyclohexanone
[0705] After 10 minutes at room temperature, under slow stirring, pyrazine-2-carbaldehyde [or 3-aminopyrazine-2-carbaldehyde; 6-amino-5-hydroxypyrazine-2-carbaldehyde; 5-hydroxy- 6-methoxypyrazine-2-carbaldehyde; 3-thiocyanatopyrazine-2-carbaldehyde; 5-(chloromethylthio)pyrazine-2-carbaldehyde; 5-acetylpyrazine-2-carbaldehyde; 6-(trifluoromethoxy)pyrazine-2-carbaldehyde; 5-fluoropyrazine-2-carbaldehyde; 5-(methylthio)pyrazine-2-carbaldehyde; 5-(chloromethylthio)pyrazine -2-carbaldehyde; 6-tert-butoxypyrazine-2-carbaldehyde; 5-acetylpyrazine-2-carbaldehyde; 6-trifluoromethoxypyrazine-2-carbaldehyde; 6-amino-5-hydroxypyridine oxazine-2-carbaldehyde; 5-hydroxy-6-methoxypyrazine-2-carbaldehyde; or 6-(trifluoromethyl)pyrazine-2-carbaldehyde: (4.62mmol)] in pure ethanol (1ml) A solution of NaOH (0.75 mmol) and cyclohexanone in pure ethanol and H was added dropwise 2 o 2 (14ml; 1:1 ratio) of the mixture in solution. The solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com