A kind of aripiprazole sustained-release microspheres and preparation method thereof
A technology of aripiprazole and sustained-release microspheres, which is applied in the field of aripiprazole sustained-release microspheres and its preparation, can solve problems such as difficult timely control of adverse drug reactions and complicated preparation techniques, and achieve improved compliance and enhanced Efficacy, the effect of reducing the frequency of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
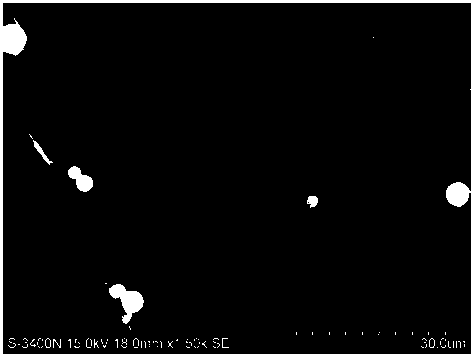
[0027] Take by weighing 220.51mgPLGA (50 / 50-2A, molecular weight 25000 Daltons), 73.39mg aripiprazole, add 4mL methylene chloride and acetonitrile (3:1) in the mixed organic solvent, stir and dissolve, form oily phase (O ), the oil phase was quickly added dropwise to 100mL containing 1.5% PVA water phase (W), stirred and emulsified at a high speed of 10000rpm for 90s to form an O / W emulsion, and then the water phase was diluted by one time with purified water, and the obtained The O / W type emulsion was placed on a magnetic stirrer at 800 rpm and stirred overnight to volatilize and remove the organic solvent to solidify the microspheres, collect the microspheres by centrifugation, wash with purified water and freeze-dry in vacuum to obtain aripiprazole microspheres. The drug loading capacity is 20.63%, and the encapsulation efficiency is 82.52%.
Embodiment 2~7
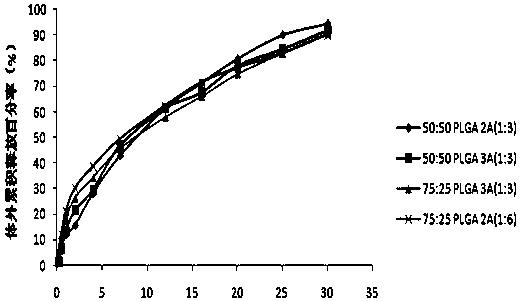
[0029] See Table 1 for the prescription.
[0030] The prescription of table 1 embodiment 2~7 slow-release microspheres
[0031]
[0032] The microsphere preparation step of embodiment 2~7 is the same as embodiment 1
Embodiment 8~14
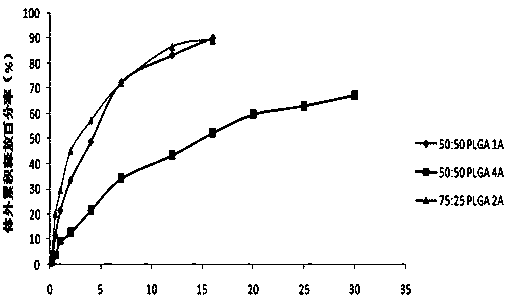
[0034] The prescription is shown in Table 2.
[0035] The prescription of table 2 embodiment 8~14 slow-release microspheres
[0036]
[0037] The microsphere preparation step of embodiment 8~14 is the same as embodiment 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com