A kind of 1,5-dicarbonyl derivative and preparation method thereof
A derivative and dicarbonyl technology, applied in the field of 1,5-dicarbonyl derivatives and their preparation, can solve problems such as poor diastereoselectivity, and achieve the effects of complex and diverse structures and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A kind of 1,5-dicarbonyl derivatives, its structural formula is:
[0041]
[0042] A kind of preparation method of 1,5-dicarbonyl compound, comprises the following steps:
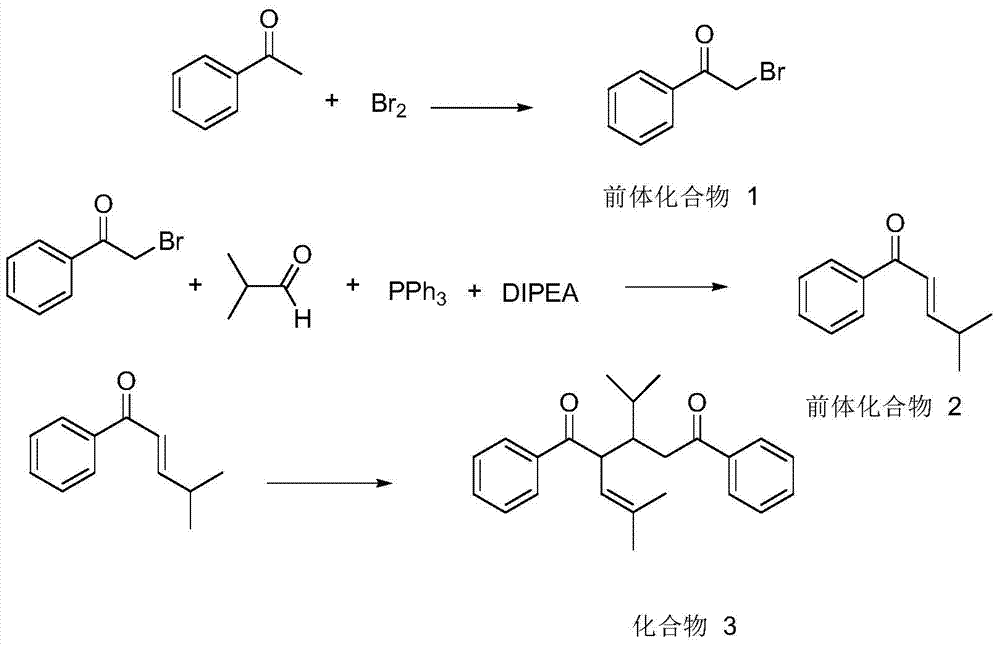
[0043] (1) At 0°C, add 50g of acetophenone and 50mL of anhydrous ether into a dry three-necked flask; install a stirrer, dropping funnel and reflux condenser on the three-necked flask; Aluminum trichloride water, add 0.42mol bromine from the dropping funnel;
[0044] After adding bromine, immediately spin dry under reduced pressure to remove diethyl ether and generated hydrogen bromide, and a brownish-yellow solid is precipitated; then add a mixture of 10 mL of water and 10 mL of petroleum ether to the reaction flask, shake to remove the color, and filter to obtain a crude product. The crude product was recrystallized from methanol to give white 2-bromoacetophenone. That is, compound 1; such as image 3 Shown in the first step reaction.
[0045] (2) Add 1mol of compound 1, 2.2mol of isobutyral...
Embodiment 2
[0052] A kind of 1,5-dicarbonyl derivatives, its structural formula is:
[0053]
[0054] A kind of preparation method of 1,5-dicarbonyl compound, comprises the following steps:
[0055] (1) At 0°C, add 64.1g of p-chloroacetophenone and 50mL of anhydrous ether into a dry three-necked bottle; install a stirrer, dropping funnel and reflux condenser on the three-necked bottle; after starting stirring, add 0.5g anhydrous aluminum trichloride, add 0.42mol bromine from the dropping funnel;
[0056] After adding bromine, immediately spin dry under reduced pressure to remove diethyl ether and generated hydrogen bromide, and a brownish-yellow solid is precipitated; then add a mixture of 10 mL of water and 10 mL of petroleum ether to the reaction flask, shake to remove the color, and filter to obtain a crude product. The crude product was recrystallized from methanol to give white 2-bromo-p-chloroacetophenone. That is, compound 1; such as Figure 5 Shown in the first step reaction. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com