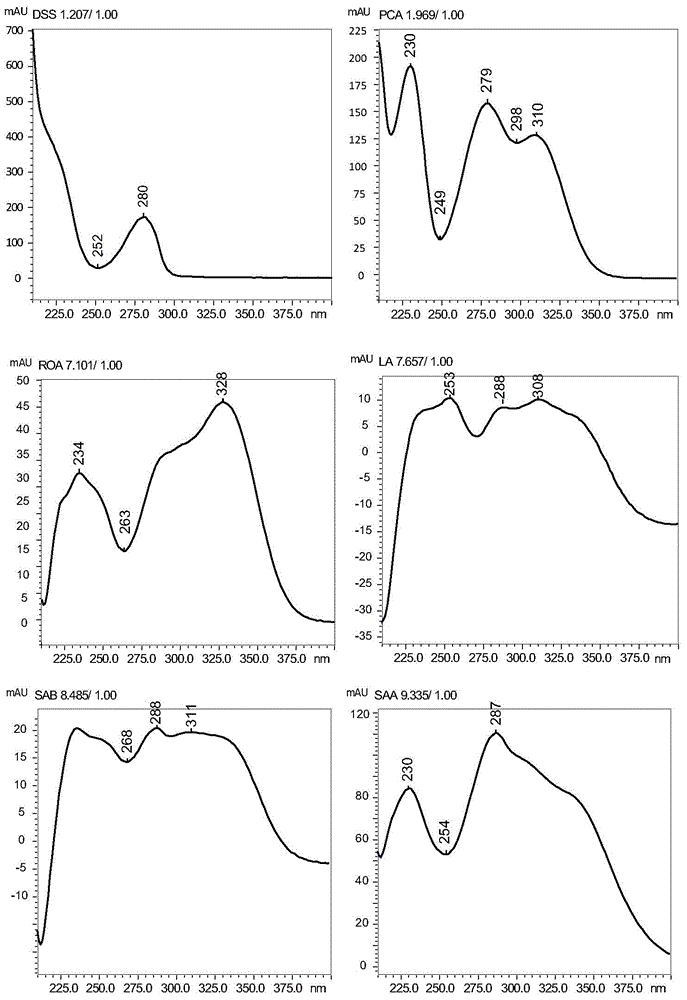
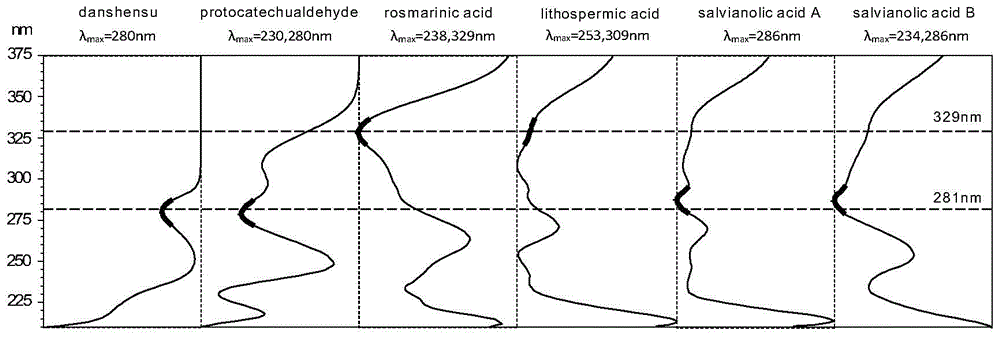
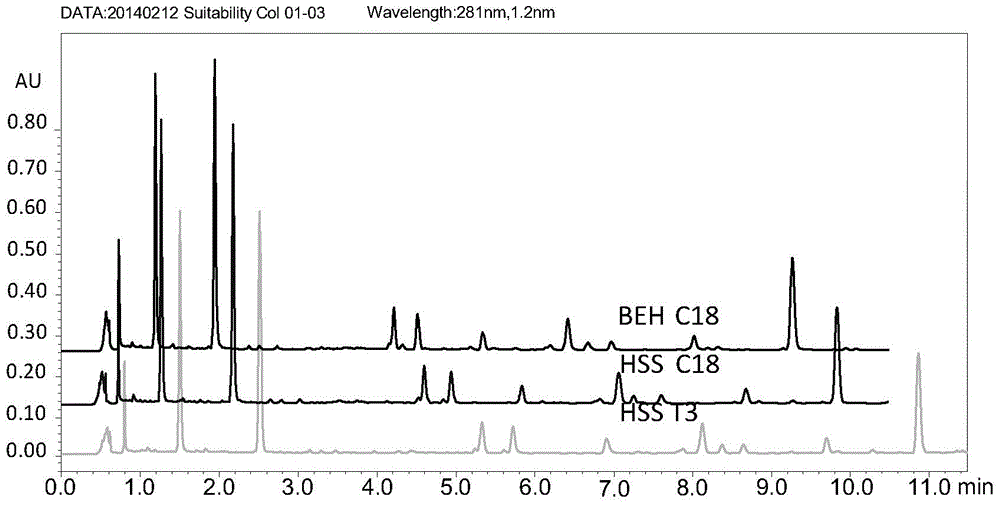
Detection method for compound radix salviae miltiorrhizae dripping pills through quantitative analysis of multiple components by single marker
A technology of compound salvia miltiorrhiza and detection methods, applied in the direction of measuring devices, instruments, scientific instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0274] 1) Compound Danshen dripping pills are prepared into test solution;
[0275] Take 25 capsules of Compound Danshen Dripping Pills, weigh them accurately, add 20mL of di-pure water to a 25mL measuring bottle, ultrasonically (300W, 50kHz) for 10min, wait to cool to room temperature, dilute to 25mL, pass through a 0.22μm filter membrane, and wait for Test samples.
[0276] 2) Determining the protocatechualdehyde content in the test solution with an ultra-high performance liquid chromatograph;
[0277] Take an appropriate amount of protocatechuic aldehyde reference substance, weigh it accurately, and prepare a reference solution with a concentration of about 40 μg / mL
[0278] Sample determination, using UPLC to determine the peak area of the analyte in the sample solution and the reference solution,
[0279] Chromatographic conditions
[0280] Chromatographic column: ACQUITYUPLCBEHC18 (1.7μm, 2.1mmi.d.×100mm), WatersACQUITYUPLCBEHC18 pre-column (1.7μmparticles, 2.1mmi.d...
Embodiment 2
[0297] 1) Compound Danshen dripping pills are prepared into test solution;
[0298] Take 25 capsules of Compound Danshen Dripping Pills, weigh them accurately, add 20mL of di-pure water to a 25mL measuring bottle, ultrasonically (300W, 50kHz) for 10min, wait to cool to room temperature, dilute to 25mL, pass through a 0.22μm filter membrane, and wait for Test samples.
[0299] 2) Determining the protocatechualdehyde content in the test solution with an ultra-high performance liquid chromatograph;
[0300] Use ultra-high performance liquid chromatography to measure the peak areas of protocatechualdehyde in the sample solution to be tested and the reference substance solution, and calculate the content of protocatechualdehyde by the working curve method; five gradients of protocatechualdehyde in the concentration range of 10-100 μg / mL are used. Catechin aldehyde was used as the reference solution.
[0301] Column:; or DikmaEndeavorsil column
[0302] Mobile phase selection:
...
Embodiment 3
[0317] 1) Compound Danshen dripping pills are prepared into test solution;
[0318] Compound Danshen dripping pills were prepared into a test solution; the method was as follows: Take 30 compound Danshen dripping pills, weigh them accurately, add 25mL water into a 30mL measuring bottle, and ultrasonically 15min, wait to cool to room temperature, dilute to 30mL, pass through 0.3 μm filter membrane, the filtrate obtained is the liquid to be tested
[0319] 2) Determining the protocatechualdehyde content in the test solution with an ultra-high performance liquid chromatograph;
[0320] Use ultra-high performance liquid chromatography to measure the peak area of protocatechualdehyde in the sample solution to be tested and the reference substance solution respectively, and use the external standard one-point method, wherein the external standard one-point method uses protocatechualdehyde with a concentration of 40 μg / mL as the reference substance ,
[0321] Chromatographic colu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com