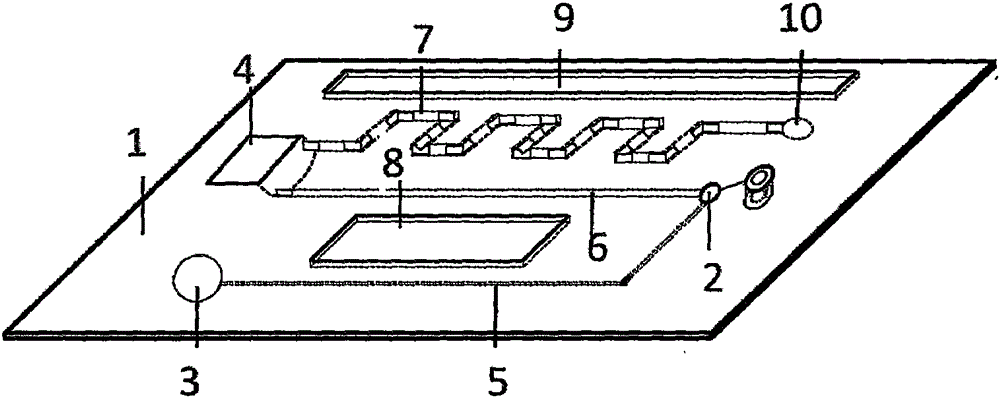
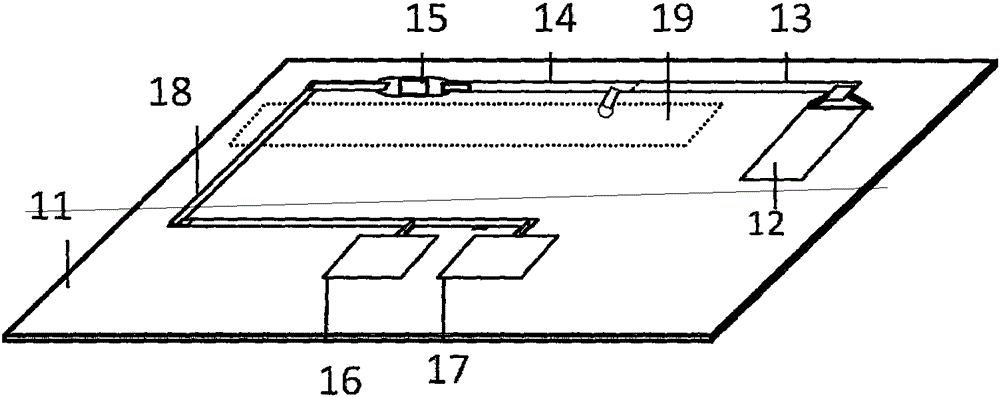
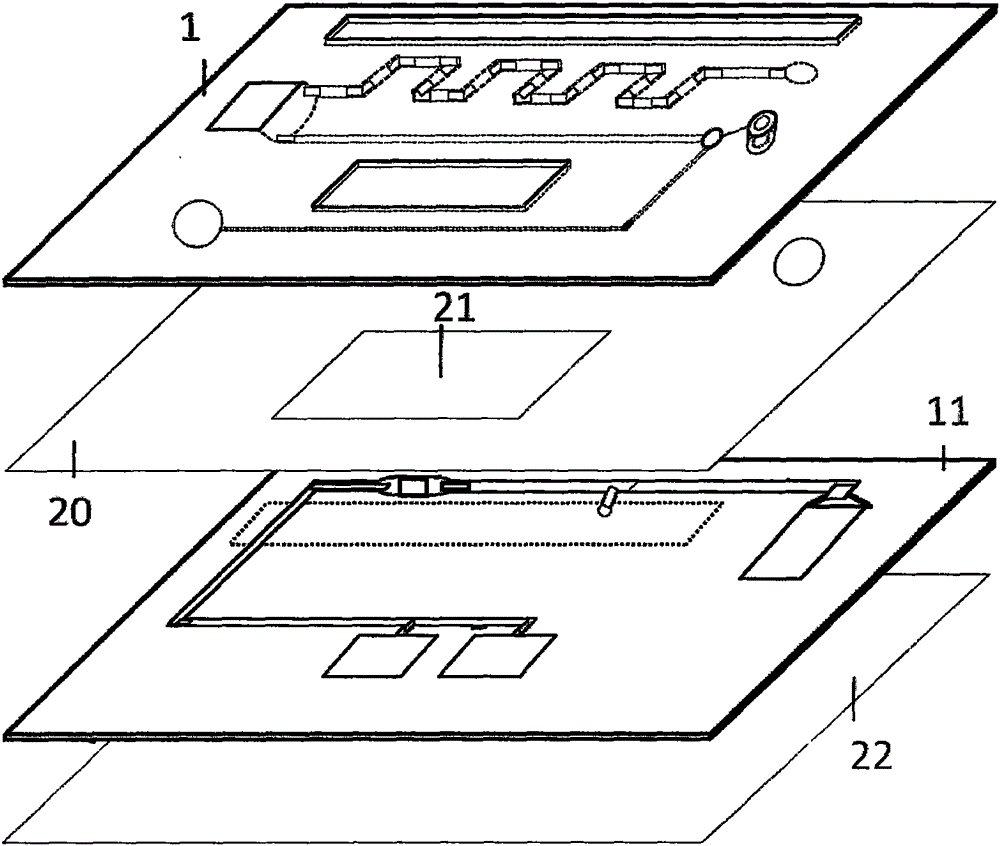
Micro-fluidic chip for multi-object quantitative detection based on magnetic particle chemiluminescence
A microfluidic chip and quantitative detection technology, applied in the direction of chemiluminescence/bioluminescence, chemical instruments and methods, and analysis through chemical reactions of materials, etc., can solve the high cross-contamination rate of reagents and the large consumption of reagents and samples , low sensitivity and repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1. Troponin I (cTnI), myoglobin (Myo), creatine kinase isoenzyme (CK-MB) joint detection method one
[0053] Three items of myocardial infarction, ie troponin I (cTnI), myoglobin (Myo) and creatine kinase isoenzyme (CK-MB), are detected simultaneously by using the method of the present invention. To complete the project test, the specific steps are as follows:
[0054] 1. Encoding of Magnetic Particle Groups
[0055] Three kinds of magnetic particles with a particle size of 2.0 μm and a magnetic content of 0.5%, 5%, and 30% were selected, respectively labeled 1, 2, and 3, which were respectively used for the detection of cTnI, Myo, and CK-MB.
[0056] 2. Antibody labeling:
[0057] 1) Enzyme-labeled antibody: Weigh 5 mg of HRP and dissolve it in 1 ml of distilled water to make a 5 mg / ml HRP solution, add 0.2 ml of newly prepared 0.1M NaIO to the solution 4 The solution was stirred at room temperature in the dark for 20 minutes; the solution was put into a ...
Embodiment 2
[0068] Example 2. Combined detection method for troponin I (cTnI), myoglobin (Myo), and creatine kinase isoenzyme (CK-MB)
[0069] Three items of myocardial infarction, ie troponin I (cTnI), myoglobin (Myo) and creatine kinase isoenzyme (CK-MB), are detected simultaneously by using the method of the present invention. Troponin I (cTnI), myoglobin (Myo) and creatine kinase isozyme (CK-MB). To complete the project test, the specific steps are as follows:
[0070] 1. Encoding of Magnetic Particle Groups
[0071] Select three kinds of magnetic particles whose magnetic content is 10%, but the particle size of the magnetic particles is 0.5 μm, 2.0 μm, and 5.0 μm, respectively, labeled 1, 2, and 3, respectively, corresponding to the detection of cTnI, Myo, and CK-MB .
[0072] 2. Antibody labeling:
[0073] 1) Enzyme-labeled antibody: Weigh 5 mg of HRP and dissolve it in 1 ml of distilled water to make a 5 mg / ml HRP solution, add 0.2 ml of newly prepared 0.1M NaIO to the solution...
Embodiment 3
[0084] Example 3: Magnetic Particle Size Screening
[0085] The particle size of magnetic microspheres is small, the specific surface area is large, and the surface contains active groups, so the coupling capacity is large, but the size of magnetic particles is too small to be conducive to magnet collection, so the magnetic particle size screening is carried out.
[0086] For other experimental conditions, refer to Example 2. The content of magnetic particles is 10%, and the size is determined according to the following scheme.
[0087] Six kinds of magnetic particle labels with particle sizes of 0.1 μm, 0.5 μm, 1 μm, 2 μm, 3 μm and 10 μm were selected to mark anti-troponin I (cTnI), myoglobin (Myo), creatine kinase isoenzyme (CK) -MB) antibodies for separate determinations of the three targets. The permanent magnet whose magnetic size has been optimized is used for detection, and the height and movement speed of the magnet are fixed.
[0088] The results of the separate det...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com