A monodisperse cyclic azobenzene-tetraethylene glycol copolymer and its preparation method and use
A technology of cyclic azobenzene and monodispersity, which is applied in the field of polymer materials and can solve the problems of few reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1: Preparation of the first generation bromine-containing intermediate and chain extender.
[0076] 1. Preparation of tetraethylene glycol intermediate (Br-TEG-OH).
[0077] Take 3.88 g (20 mmol) of tetraethylene glycol, 1.4 mL (20 mmol) of triethylamine and 10 mL of dry dichloromethane and place them in a 50 mL three-necked flask equipped with a stirring device, cool in an ice-salt bath and control the temperature to 0~5℃. Dissolve 3 mL (20 mmol) of 6-bromohexanoyl chloride in 5 mL of dry dichloromethane, and add it dropwise into a three-necked flask with a constant pressure dropping funnel, controlling the solution temperature at 0-5 °C. After the dropwise addition was completed, the reaction was carried out at room temperature for 2.5 h. After the reaction was completed, it was suction filtered, and the filtrate was washed with dilute hydrochloric acid, 1M NaOH and saturated NaCl aqueous solution to pH=7. The obtained organic phase was dried over anhydrous...
Embodiment 2
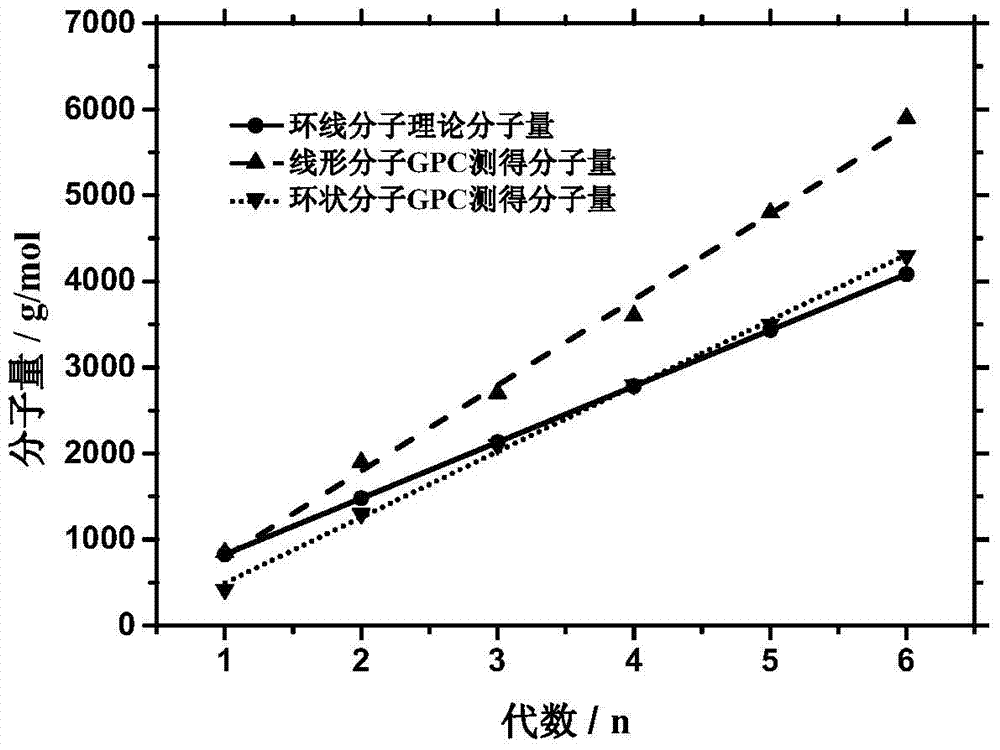
[0094] Embodiment 2: Preparation of the 2nd to 6th generation bromine-containing intermediates.
[0095] 1. The second generation of bromine-containing intermediates (α-alkynyl-(Azo-TEG) 2 -ω-bromo).
[0096] Add 1.82 g (2.5 mmol) of the first-generation bromine-containing intermediate (α-alkynyl-(Azo-TEG) 1 -ω-bromine) and 20 mL of dry dichloromethane, after argon for 30 min, add 35.8 mg (0.25 mmol) CuBr and 0.06 mL (0.25 mmol) PMDETA, slowly add 1.82 g (3 mmol) dropwise under the protection of argon ) chain extender (TMS-alkynyl-Azo-TEG-azide), react at room temperature for 1 h after the dropwise addition, and obtain the second-generation bromine-containing intermediate protected by TMS; then add 0.4 mL (0.8 mmol ) TBAF, stirred at room temperature for 1 h, then poured into ethyl acetate, extracted three times with deionized water, dried the organic layer with anhydrous magnesium sulfate overnight, filtered with suction, rotary evaporated, and purified by column chromatogr...
Embodiment 3
[0106] Embodiment three: the preparation of linear copolymer intermediate and cyclic copolymer.
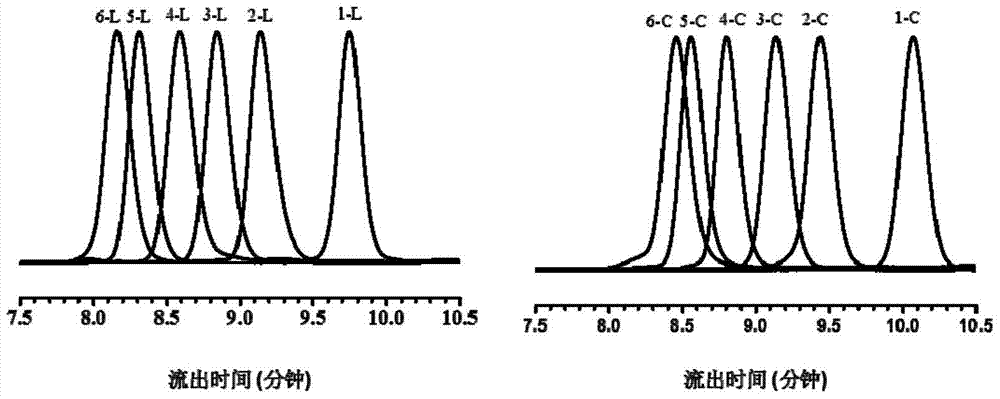
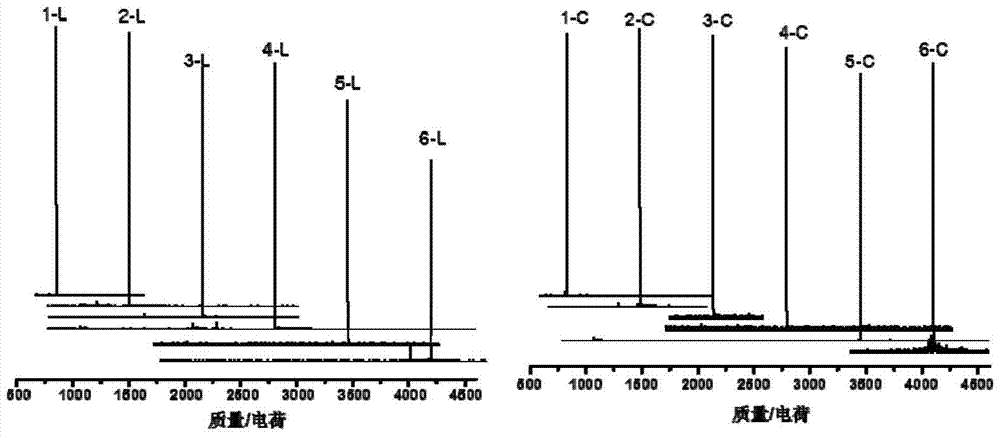
[0107] 1. The first generation of linear copolymer intermediates ( l -(Azo 2 -TEG 1 )) and cyclic copolymers ( c -(Azo 2 -TEG 1 )) preparation.
[0108] (a) l -(Azo 2 -TEG 1 ) preparation: take 0.548 g (1 mmol) α-alkynyl-(Azo-TEG) 1 -ω-bromine, 0.552g (1 mmol) K 2 CO 3 , 0.017 g (0.1 mmol) KI and 40 mL DMF were placed in a 100 mL round bottom flask equipped with a stirring device. 0.444 g (2 mmol) of 3-alkynyl-4’-hydroxyazobenzene (HAAzo) was dissolved in 15 mL of DMF, slowly added dropwise to the reaction system, and reacted at 80 °C for 4 h. The reaction solution was naturally cooled to room temperature, then poured into ethyl acetate, extracted three times with deionized water, dried the organic layer with anhydrous magnesium sulfate overnight, filtered with suction, rotary evaporated, and purified by column chromatography to obtain l -(Azo 2 -TEG 1 ) (yield 0.82...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com