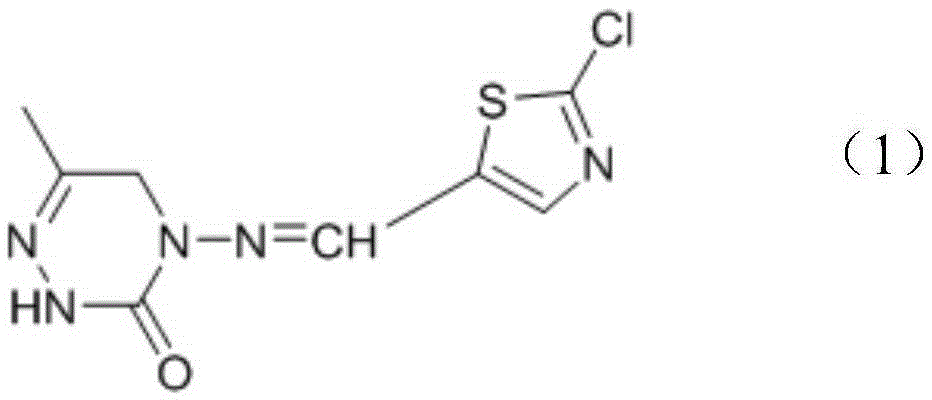
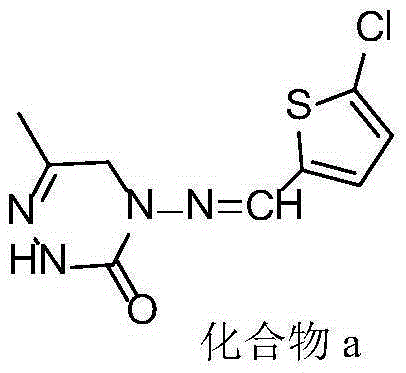
Triazinone compound containing thiazole rings and preparation method and application thereof
A triazinone-containing technology containing a thiazole ring, applied in the field of agricultural and forestry insecticides, can solve the problems of no obvious lethality and resistance of moth pests, achieve good insecticidal activity, solve the problem of resistance, and high The effect of insecticidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
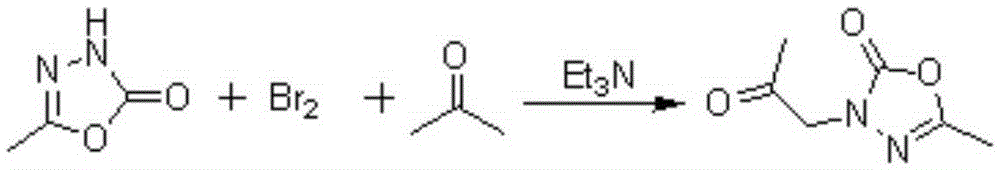
[0028] Example 1: Preparation of 2-methyl-4-acetonyl-1,3,4-oxadiazol-5-one
[0029] Add 49.6g of bromine dropwise to 85mL of acetone, after the solution becomes clear, add 90mL of triethylamine and stir for 30min, filter off triethylamine hydrogen bromide, and then add 29g (0.3mol) of 2-methyl- 1,3,4-Oxadiazol-5(4H)-one was added into the solution and refluxed for 3 hours. Desolvation gave 43g of 2-methyl-4-acetonyl-1,3,4-oxadiazol-5-one.
Embodiment 2
[0030] Example 2: Preparation of 4-amino-6-methyl-3-ketone-2,5,5-trihydro-1,2,4-triazine
[0031] Dissolve 33g (0.2mol) of 2-methyl-4-acetonyl-1,3,4-oxadiazol-5-one in 40mL of methanol, add 13g of 80% hydrazine hydrate dropwise at room temperature, reflux for 3 hours after dropping, and evaporate Remove most of the solvent, cool in an ice bath to 10°C, slowly drop into 19.5g of concentrated hydrochloric acid, react at 40°C for 4 hours, cool, and remove the solvent to obtain 17.9g of 4-amino-6-methyl-3-ketone-2, 5,5-Trihydro-1,2,4-triazine.
Embodiment 3
[0032] Embodiment 3: Preparation of 2-chloro-5-formaldehyde base thiazole
[0033] Add 33.6g (0.24mol) of hexamethylene iminium to 303g of chloroform, heat to reflux, then slowly add 32.4g (0.2mol) of 2-chloro-5-chloromethylthiazole dropwise, keep reflux after dropping 3 hours. After cooling to below 0°C with an ice bath, the obtained solid was first dissolved with 17% hydrochloric acid solution and stirred for 30 minutes, then added with 48% glacial acetic acid and heated to 60-70°C for 3 hours. Then 3×50 mL of ethyl acetate was added for extraction, and the extract was washed with 3% aqueous sodium carbonate solution, dried and precipitated to obtain 21.1 g of light yellow solid. IR(KBr) displayed at 1674cm -1 There is a strong absorption peak.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com