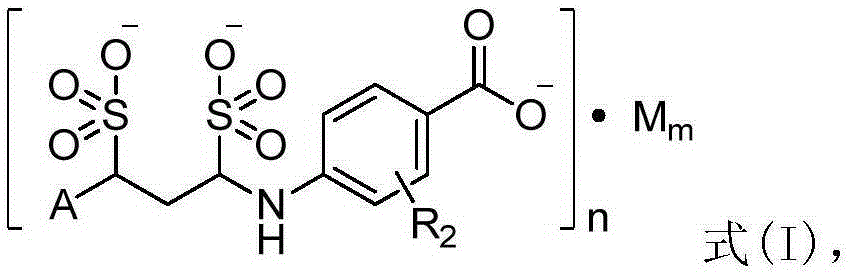
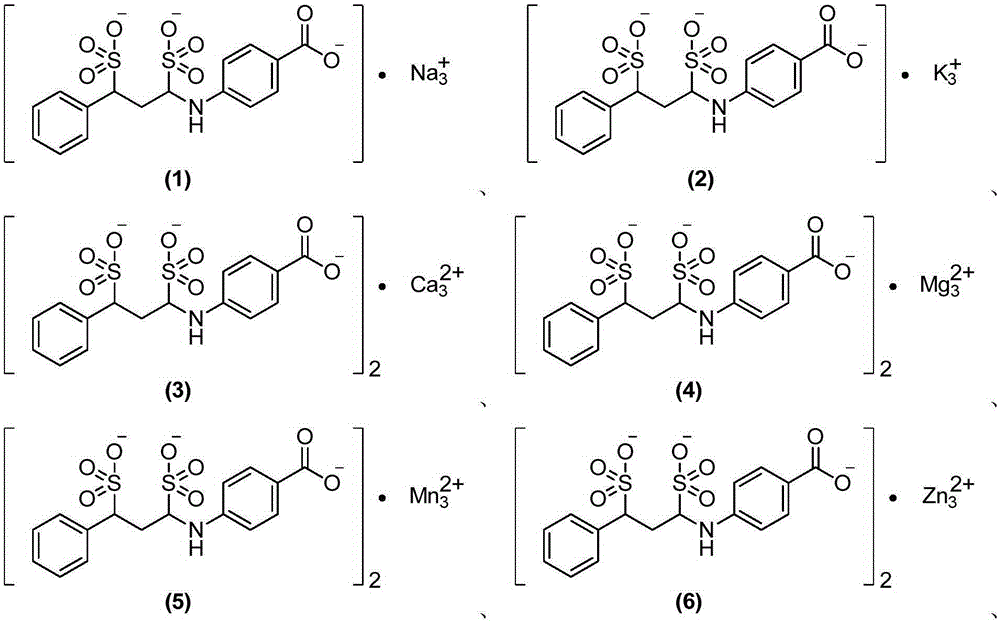
Para aminobenzoic acid derivatives and composition and application thereof
A technology of para-aminobenzoic acid and derivatives, applied in the application, animal feed, sulfonate preparation and other directions, can solve the problem of carcinogenicity, the stability of feed biocide and the difficulty in production, sales and management, and the impact on human reproductive performance. and sexuality issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0111] Example 14-((E)-phenylene imino)benzoic acid sodium salt
[0112]
[0113] In a 1L three-necked round-bottom flask, add 220 mL of 95% ethanol and 4.2 g of sodium hydroxide (96%, 100 mmol, 1 eq) in sequence, and stir mechanically until the sodium hydroxide dissolves. After the above sodium hydroxide solution was cooled to room temperature, 13.7g (100mmol, 1eq) of p-aminobenzoic acid was added, stirred and dissolved, and 13.2g (100mmol, 1eq) of 3-phenylacrolein was added, stirred overnight at room temperature to obtain a yellow suspension, TLC Monitor until the end of the reaction, filter with suction, wash the filter cake with 95% ethanol (30mL×3), and dry under reduced pressure at 55°C to obtain 12.6g of (4-((E)-phenyleneimino)benzoic acid sodium salt as a yellow powder solid ), yield 46.2%.
[0114] The NMR data of 4-((E)-phenyleneimino)benzoic acid sodium salt are as follows:
[0115]1 HNMR (500MHz, DMSO-d 6 ): δ(ppm)8.39(d,J=9.0Hz,1H),7.83(d,J=8.0Hz,1H),7.67(d,...
Embodiment 24
[0116] Example 24-(1,3-disulfonic acid phenylpropylamino)benzoic acid sodium salt (1:3)
[0117]
[0118] Add 200mL of water and 273.1g (100mmol, 1eq) of 4-((E)-phenylpropyleneimino)benzoic acid sodium salt into a 1L three-necked round-bottomed flask in turn, stir mechanically to disperse evenly, and add sodium bisulfite to the reaction solution 20.8g (200mmol, 2eq), stirred and reacted at room temperature for 8h to obtain a clear reaction solution. Add 200 mL of 95% ethanol to the obtained clarified reaction solution and stir at room temperature to obtain a white suspension, filter under reduced pressure, wash the filter cake with 95% ethanol (30 mL×3), and dry under reduced pressure at 55°C to obtain the target product as a white powder Solid (4-(1,3-disulfonic acid phenylpropylamino) benzoic acid sodium salt) 31.6g, yield 65.7%.
[0119] 1 HNMR (500MHz, DMSO-d 6 ): δ (ppm) 7.47 (d, J = 8.0Hz, 2H), 7.17 (m, 5H), 6.22 (d, J = 8.5Hz, 2H), 5.21 (d, J = 10.0Hz, 1H), 3.62 ...
Embodiment 34
[0120] Example 34-(1,3-disulfonic acid phenylpropylamino)benzoic acid potassium salt (1:3)
[0121]
[0122] In a 1L three-necked round-bottomed flask, 220 mL of ethanol and 5.8 g of potassium hydroxide (96%, 100 mmol, 1 eq) were sequentially added, and mechanically stirred until the potassium hydroxide was dissolved. Add 13.7g (100mmol, 1eq) of p-aminobenzoic acid to the above potassium hydroxide solution, stir to dissolve, add 13.2g (100mmol, 1eq) of cinnamaldehyde, stir overnight at room temperature, a large amount of solids in the reaction solution precipitate to form a suspension, pump Filter and dry under vacuum at 40°C. The obtained product was dissolved in 200 mL of water, 24.0 g (200 mmol, 2 eq) of potassium bisulfite was added, and the reaction was stirred at room temperature for 8 h to obtain a clear reaction liquid. Add 1L of ethanol to the obtained clarified reaction solution and stir at room temperature to obtain a white suspension, filter under reduced press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com