Ornithine- or aspartate-containing compositions and the uses thereof
A technology of aspartic acid and composition, applied to the composition containing ornithine and/or aspartic acid and its application field, capable of solving problems such as symptoms of weakness, increased blood sugar level, rhabdomyolysis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0101] The preparation methods and compositions described herein can be used to reduce the level of one or more risk factors for hyperlipidemia in serum. In certain embodiments, the methods and compositions can be used to reduce total cholesterol, triglycerides, or low-density lipoprotein levels; in certain embodiments, the methods and compositions can be used to reduce CPK levels in an individual; In certain embodiments, the methods and compositions are useful for increasing high-density lipoprotein levels in an individual; in certain embodiments, the methods and compositions are useful for reducing serum glucose levels; abnormal hyperlipidemia risk factors A level means that the risk factors for hyperlipidemia in the individual's serum are beyond the range generally expected by those skilled in the art. General expected ranges for total cholesterol, LDL, HDL, and triglyceride levels are defined by the National Heart, Lung, and Blood Institute, National Institutes of Health (...
example 1
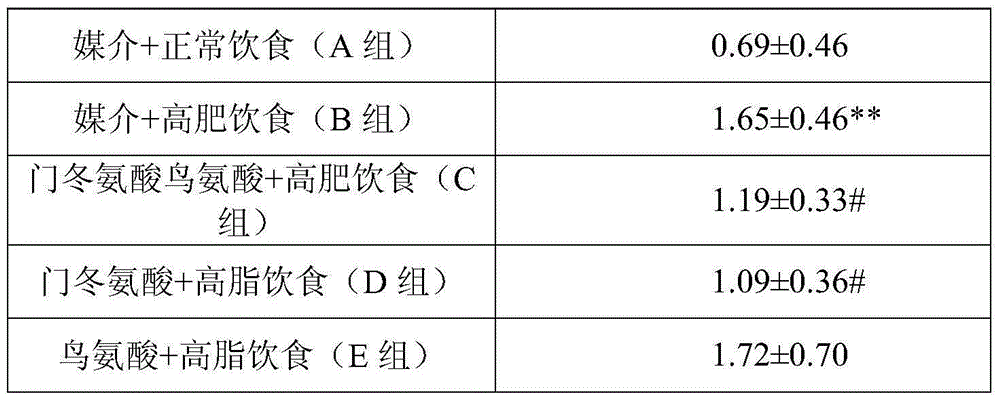
[0122] Example 1: Effect of Ornithine and / or Aspartate on Rat Plasma Triglycerides and CPK Activity
[0123] This trial conducted a study on the use of a composition comprising ornithine and / or aspartate, which also includes ornithine aspartate, for the treatment of hyperlipidemia in individuals Hyperemia, lowering triglyceride levels. In addition, the administration of lipid-lowering drugs such as atorvastatin may cause the increase of CPK in plasma, and the composition containing ornithine-aspartate may reduce the increase of CPK, and we have also conducted related research accordingly.
[0124] Male Wistar rats (purchased from Beijing Huafukang Co., Ltd., Beijing, China), each weighing about 180-250g, were divided into five groups and raised in animal rooms with good ambient temperature (about 21±1°C) and humidity control. Give lighting 12h every day and dark avoiding light (lighting starts at 7:00 a.m.), cycle feeding, diet and water intake are not limited, and the food c...
example 2
[0139] Example 2: The formula and preparation process of ornithine-aspartate tablet
[0140]Compositions containing ornithine and / or aspartic acid can be prepared in the form of tablets. This experiment describes the preparation process of ornithine-aspartate tablets. The formulation of the tablets is shown in Table 3.
[0141] First mix 302.7g of povidone, 0.5g of sweet orange essence, and 0.0025g of sunset yellow pigment with 135g of 80% ethanol, and simply ultrasonically mix to prepare a viscous mixture. This viscous mixture was then added to a mixture formed by thoroughly mixing 150 g ornithine aspartate, 7.5 g citric acid, 105 g mannitol, and 2.5 g aspartame, mixed thoroughly again to form a soft mass, and then Dry, form granules and pass the granules through a 18 mesh screen. The sieved granules were added with 5 g of talcum powder and thoroughly mixed to prepare tablets.
[0142] Table 3. Formulation of ornithine-aspartate tablets
[0143] Ornithine Aspartat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com