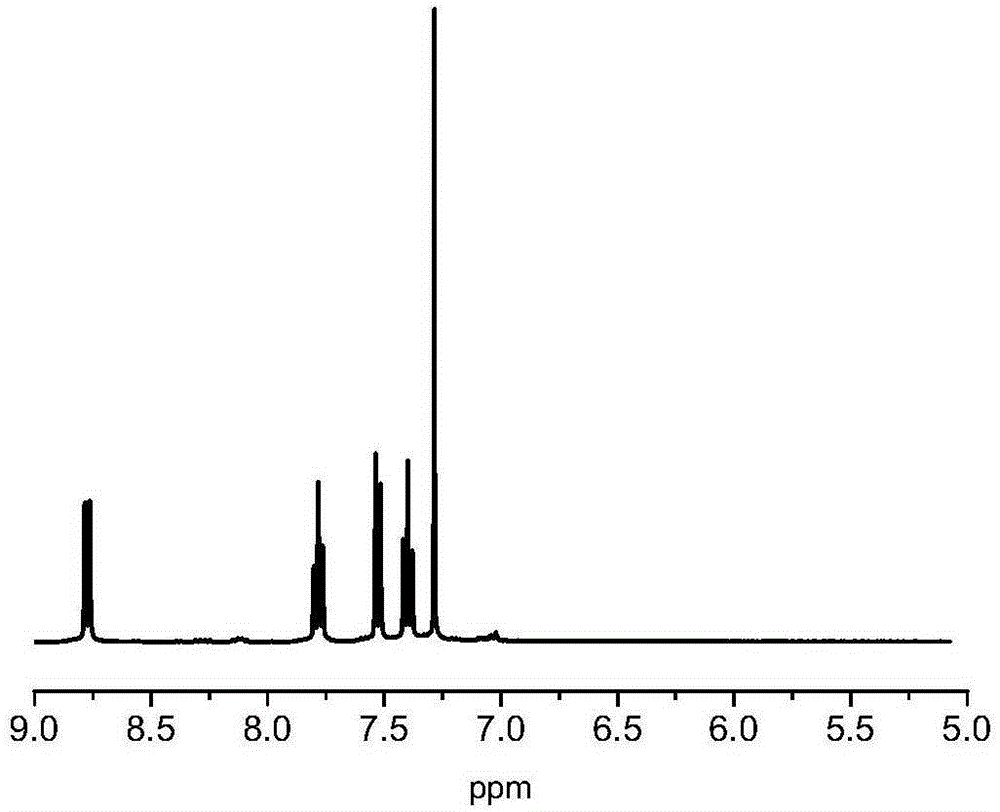
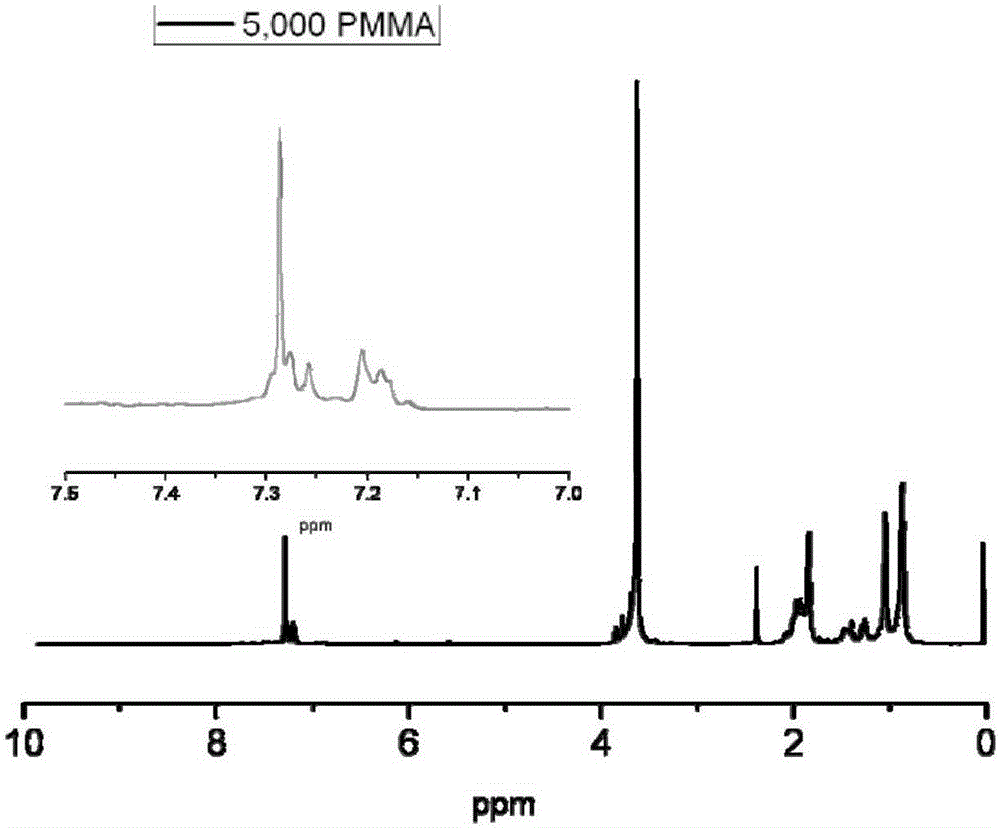
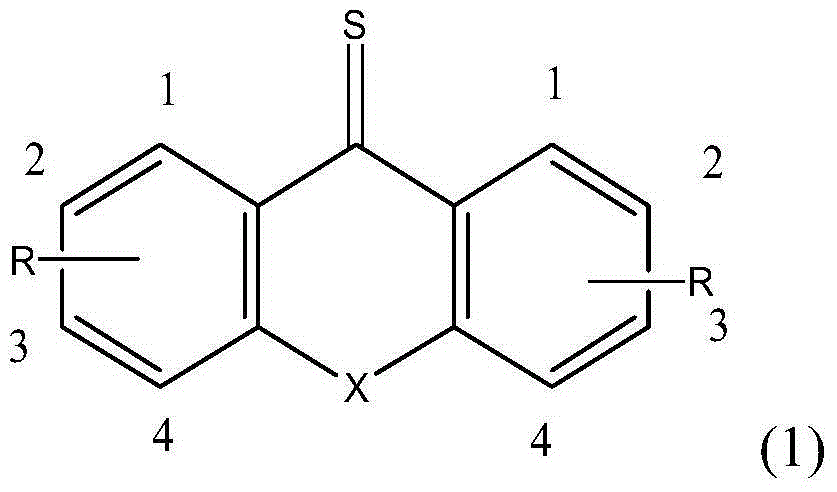
Active free radical polymerization process using aromatic annular sulfur (oxygen) impurity thioketone, derivatives of aromatic annular sulfur (oxygen) impurity thioketone and radical initiator in cooperation
A polymerization method and free radical technology, applied in the direction of organic chemistry, etc., can solve the problems of insignificant molecular weight increase, high initiation activity, low chain free radical coupling ability, etc., and achieve the effect of a wide range of applications.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Thiathione regulates MMA solution polymerization at different reaction temperatures.
[0046] In a 100ml single-necked bottle, add 0.0500g of thiathione, 0.0164g of AIBN, 5.0000g of MMA, and 15g of toluene. After sealing, freezing and thawing and degassing three times, react at 70 degrees and 80 degrees respectively for 24 hours. Take out the samples regularly and quickly place them in liquid nitrogen to quench them. After vacuum drying, the conversion rate of the monomers increases linearly with the reaction time as measured by gravimetric method; Large, the dispersion coefficient is basically below 1.8. The obtained living free radical polymer can be used as a macroinitiator to continue to initiate block copolymerization of other monomers.
[0047] The results of the 70 degree experiment are shown in Table 1.
[0048] time (hours)
Conversion rates(%)
molecular weight
polydispersity coefficient
1
27.2
41400
1.78
...
Embodiment 2
[0051] Example 2: Thiathione regulates MMA solution polymerization at different MMA concentrations.
[0052] In a 100ml single-necked bottle, add thiathione, AIBN, MMA and toluene. Add thiathione, AIBN, MMA and toluene, AIBN, thiothione mass are 0.0328g and 0.1000g respectively; MMA mass are 2.50g, 5.00g, 10.00g respectively. The total of MMA and toluene is 20.00g. Sealed, freeze-thawed and degassed three times, and reacted at 70 degrees for 24 hours. The samples were taken out regularly and quickly placed in liquid nitrogen to quench them. After vacuum drying, the monomer conversion rates of the two groups of experiments were measured by gravimetric method. The monomer conversion rate increased linearly with the reaction time; rate increases.
[0053] MMA concentration is 12.5% experimental results are shown in Table 3.
[0054] time (hours)
Conversion rates(%)
molecular weight
polydispersity coefficient
[0055] 1
8.2
16,3...
Embodiment 3
[0060] Example 3: 70-degree thiathione regulates BA solution polymerization
[0061] In a 100ml single-necked bottle, 0.0500g of thiothione, 0.0164g of AIBN, 5.00g of BA, and 15.00g of toluene. Sealed, freeze-thawed and degassed three times, and reacted at 70 degrees for 24 hours. Take out the samples regularly and quickly place them in liquid nitrogen to quench them. After vacuum drying, the monomer conversion rate increases with the prolongation of the reaction time as measured by gravimetric method; the molecular weight increases with the increase of the monomer conversion rate as observed by GPC , The dispersion coefficient is around 1.8. The obtained active polymer can be used as a macroinitiator to initiate block copolymerization of other monomers.
[0062] The experimental results are shown in Table 6.
[0063] time (hours)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com