Method and reagent kit for detecting mutation of human TERT gene promoter
A technology of promoters and kits, applied in biochemical equipment and methods, microbial measurement/inspection, etc., can solve problems such as reduced amplification efficiency, inaccurate results, and difficulty in fragment amplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 kit detection steps
[0044] 1. Refer to the following PCR 96-well plate layout table:
[0045]
[0046]
[0047] Add 10μLC228T reaction mixture to each reaction space in columns 1-4 of the 96-well plate (the volume ratio of TERTC228T forward primer, TERTC228T reverse primer and 2×PCR master mix containing fluorescent dye in TERTC228T reaction mixture is 1.25: 1.25:7.5, the concentrations of TERTC228T forward primer and TERTC228T reverse primer in TERTC228T reaction mixture are both 4.8μM), 10μLC250T reaction mixture (TERTC250T forward primer, TERTC250T reverse The volume ratio of the primer and the 2×PCR master mix containing fluorescent dye in the TERTC250T reaction mixture is 1.25:1.25:7.5, and the concentrations of the TERTC250T forward primer and the TERTC250T reverse primer in the TERTC250T reaction mixture are both 4.8 μM), 14 μL of internal standard reaction mixture was added to each reaction space in columns 9-12 (every 14 μL of TERT inter...
Embodiment 2
[0054] Embodiment 2 result judgment and analysis method
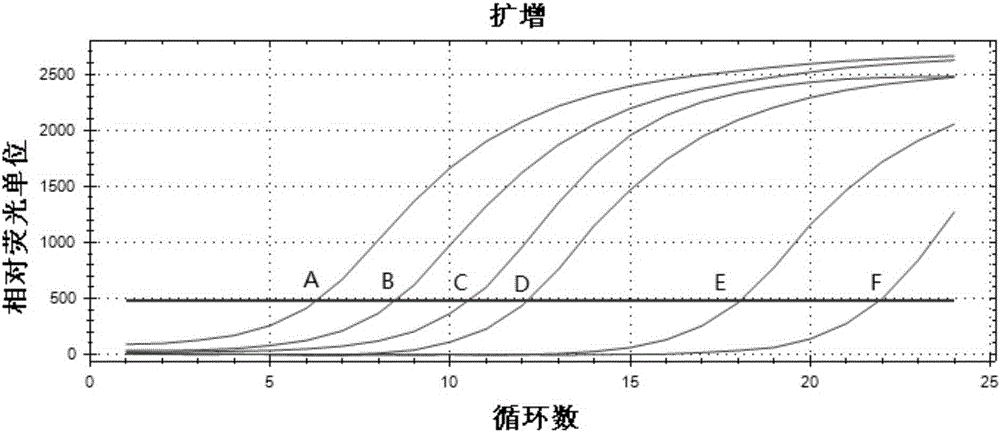
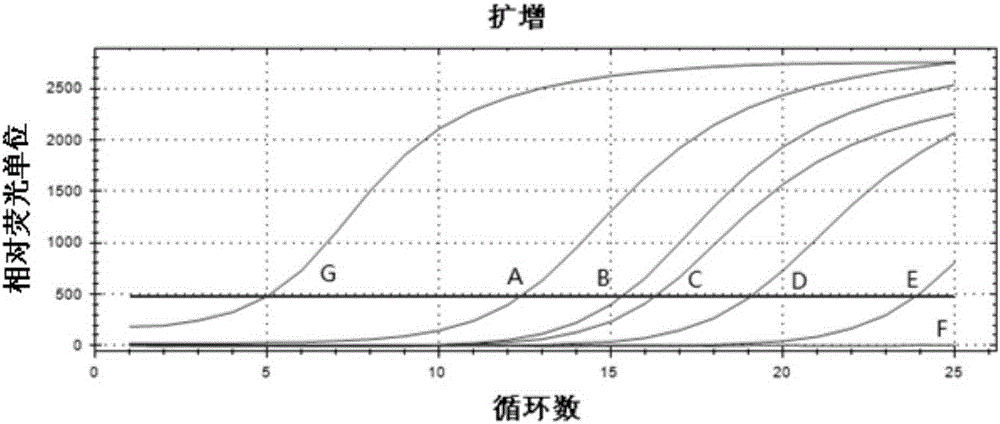
[0055] 1. Determination of mutation results: first determine the mutation Ct value (abbreviated as A) of each C228T reaction system or C250T reaction system, and then determine the Ct value of the internal standard reaction system of the sample and the Ct value of the positive quality control product. The Ct value of the internal standard reaction system plays a vital role in the calculation of the mutation ratio in the later stage. For the specific role, please refer to the next step. The Ct value of positive quality control product 1 and positive quality control product 2 can directly determine the validity of the result. It belongs to the quality control point in the reaction system. Different Ct values represent different degrees of mutation, and the detection results can be analyzed according to the result judgment criteria (Table 1):
[0056] Table 1 Results Judgment Criteria
[0057]
[0058] *Calculatio...
Embodiment 3
[0064] The optimization of embodiment 3 reaction system
[0065] 1. Confirmation of the amount of genomic DNA of the sample to be tested: add the 10ng / μl sample human genomic DNA in Example 1 to the R132H reaction system with 4 additions of 1ng, 5ng, 10ng, and 50ng respectively, and finally confirm that the concentration is 50ng (5μl). 10ng / / μl template) as the final addition amount of C228T reaction system and C250T reaction system, this addition amount can exclude the influence of human operation factors.
[0066] 2. Exploring the number of cycles: A represents the cycle number of the second stage in step 4 of embodiment 1; B represents the cycle number of the third stage in step 4 of embodiment 1, according to the following combinations, the final verification results show that A is 18 and B The collocation of 24 is the best response process.
[0067] A:18repeats(19total)B:24(23total)
[0068] A:16repeats(17total)B:26(26total)
[0069] A:14repeats(15total)B:28(29total)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com