Novel application of benzylisoquinoline alkaloid
A technology of benzyl isoquinoline and alkaloids, which is applied in the new application field of benzyl isoquinoline alkaloids to achieve the effects of inhibiting FAS expression, good clinical application prospects and inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
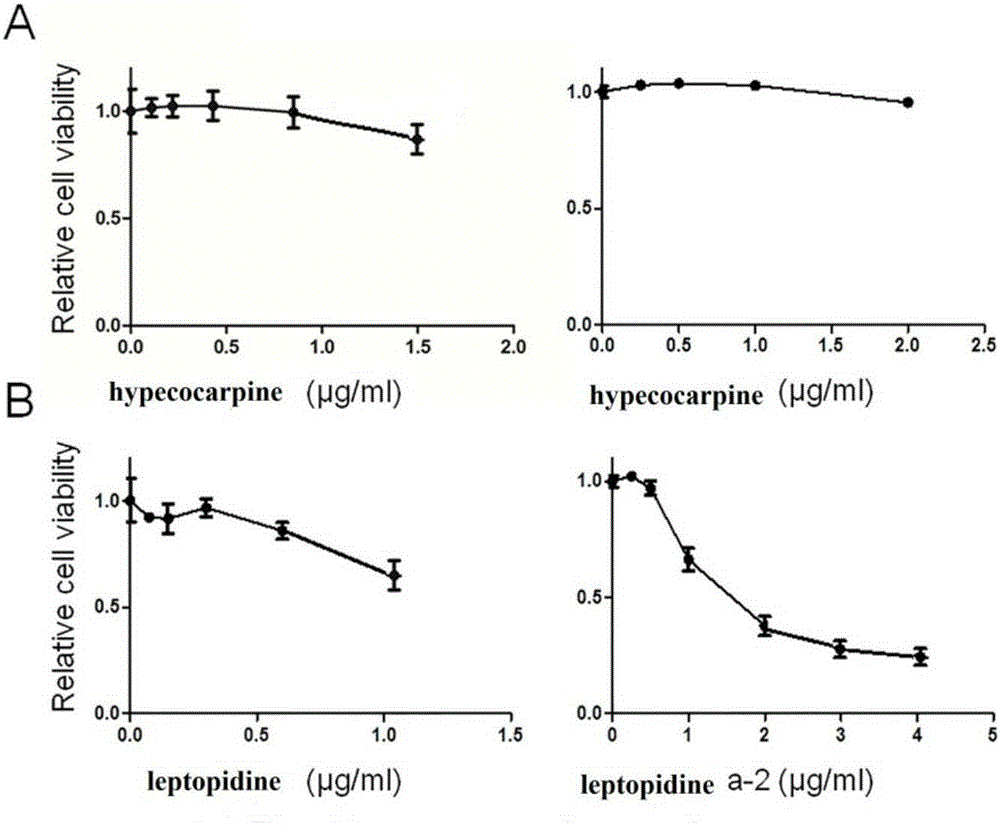
[0031] Embodiment 1 The preparation of hypococarpine and leptopidine of the present invention
[0032] The sources of experimental materials are commercially available commodities.
[0033] 1. Preparation of crude extract of fennel fennel
[0034] 2.0 kg of fennel fennel was added to 20 L of 95% ethanol to extract for 3 hours, and this was repeated 3 times. The three extracts were combined to collect the filtrate, and then concentrated by a rotary evaporator to remove ethanol to obtain an extract. The extract is dissolved with 2L of 2% hydrochloric acid, filtered, and insoluble matter is removed to obtain an acid solution of alkaloids. The acid solution was extracted with 2L petroleum ether and repeated three times to remove fat-soluble impurities. The pH value of the acid solution is adjusted to 9-10 with concentrated ammonia water. Then it was extracted with chloroform to obtain 12.6 g of alkaloid crude sample, which was used for subsequent high-speed countercurrent chro...
Embodiment 2
[0046] Embodiment 2 The purposes of hypococarpine and leptopidine of the present invention
[0047] 1. Experimental materials
[0048] The sources of experimental materials were self-made or commercially available.
[0049] 1.1 Cell lines:
[0050] Human breast epithelial carcinoma cells (MDA-MB-231): provided by Ma Xiaofeng Enzyme Laboratory, University of Chinese Academy of Sciences.
[0051] 1.2 Main reagents:
[0052] Compounds hypecocarpine and leptopidine were prepared by the method of Example 1, and the purity was greater than 97.8%; fetal bovine serum (FBS) was purchased from Beijing Sijiqing Bioengineering Materials Company; DMEM medium was purchased from Nissui Seiyaku Company in Tokyo, Japan; CCK-8 The box was produced by Tongjin Institute of Chemistry, Kumamoto City, Japan; ethylenediaminetetraacetic acid (EDTA), trypsin, and dimethyl sulfoxide (DMSO) were all purchased from Sigma-Sidrich Company; antibodies: two primary antibodies, FAS antibody and The antibod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com