A kind of method that utilizes aldehyde group-containing boron ester compound to detect peroxide
A technology of peroxides and compounds, applied in the field of fluorescent sensing materials, can solve the problems of slow, unfavorable fast detection of peroxides, non-thin-film fluorescent probes, etc., achieve high yield, avoid pretreatment process, and synthesize raw materials The effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Compound 1: Synthesis of 4-(phenyl-(4,4,5,5-tetramethyl-1,-1,3,2-boronate) phenyl) benzaldehyde (1) (wherein R1 is triphenylamine):
[0043]
[0044] Dissolve 742mg of N,N-diphenyl-4-(4,4,5,5-tetramethyl-1,-1,3,2-boronate) aniline in 4mL N,N-dimethyl In base formamide, 2 g of phosphorus oxychloride was added dropwise at room temperature, and then the reaction temperature was increased to 90° C. for 1 hour. The reaction solution was cooled to room temperature, then poured into an ice-water mixture, and extracted with dichloromethane. After the extract was spin-dried, it was separated by column chromatography to obtain 400 mg of a bright yellow solid product.
[0045] Mass spectrum (EI): m / z399
[0046] H NMR 1 H-NMR (500MHz, CDCl 3 , 25°C, TMS): δ=9.81(s, 1H), 7.76-7.75(d, 2H), 7.69-7.67(d, 2H), 7.34-7.30(m, 2H), 7.18-7.12(m, 5H ), 7.07-7.05 (d, 2H), 1.34 (s, 12H).
[0047] (2) Compound 2: 5-(9,9-dioctyl-7-(4,4,5,5-tetramethyl-1,3,2-- boronate)-9-fluorenyl ...
Embodiment 2
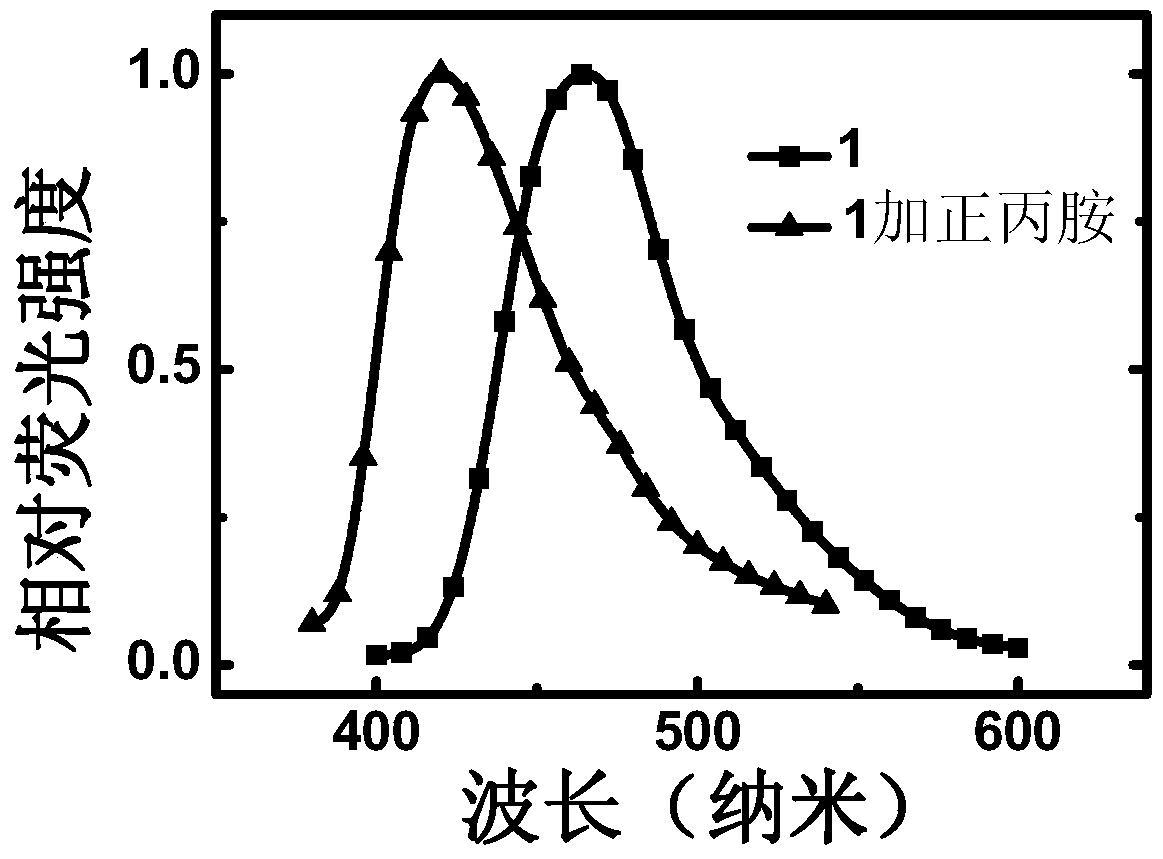
[0058] Thin films based on compound 1 were prepared on quartz wafer substrates by spin-coating method. Put a little n-propylamine (R5 is n-propyl at this time) at the bottom of the airtight container, pad a ball of absorbent cotton above it to avoid direct contact with the film, and seal the container tightly. After placing the film in a closed container for about 5 minutes, take it out, and then put it in a vacuum drying oven to dry for 5 minutes. The treated film can be used as a peroxide sensing film. The fluorescence emission spectra of the films before and after treatment were tested respectively. Such as figure 1 As shown, the maximum emission peaks of the film prepared by compound 1 and the treated sensing film are at 465nm and 420nm, respectively.
Embodiment 3
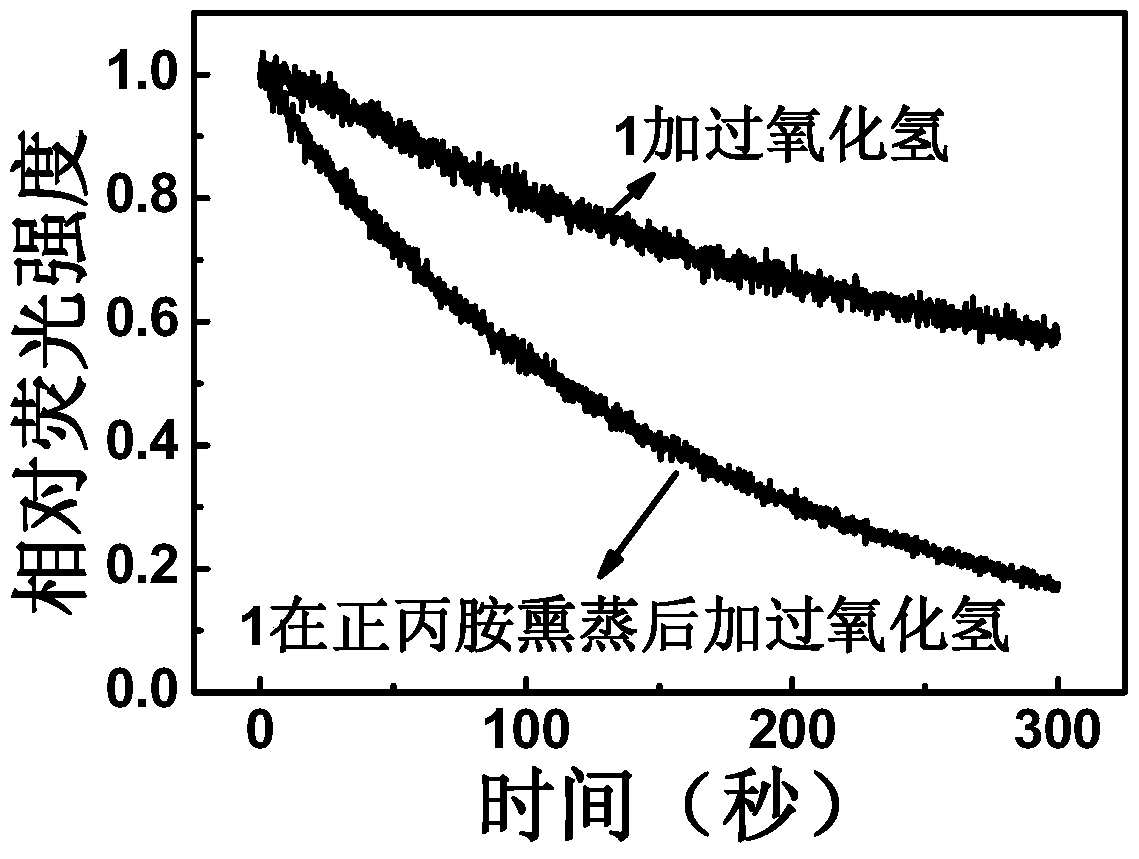
[0060] Put a drop of hydrogen peroxide on the bottom of the quartz cell, pad a ball of absorbent cotton above it to avoid direct contact with the sensing film, and seal the quartz cell with a cover. The films before and after the treatment in Example 2 were respectively placed in a closed quartz cell, and the curves of peak intensity and time at the maximum fluorescence emission peaks of 465 nm and 420 nm were measured rapidly. Such as figure 2 As shown, the intensity of the maximum emission peak of the film before and after treatment was quenched by 42% and 83% respectively within 300 seconds, which fully shows that the reaction rate of the film after primary amine treatment is accelerated and has a more sensitive response. characteristic.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com