Camptothecin phospholipid compound, drug composition and application thereof
A technology of phospholipid compound and camptothecin, which is applied in the field of medicine, can solve the problems of slow drug release, difficulty in reaching effective drug concentration, disappearance of anti-tumor activity, etc., and achieve the effect of low toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
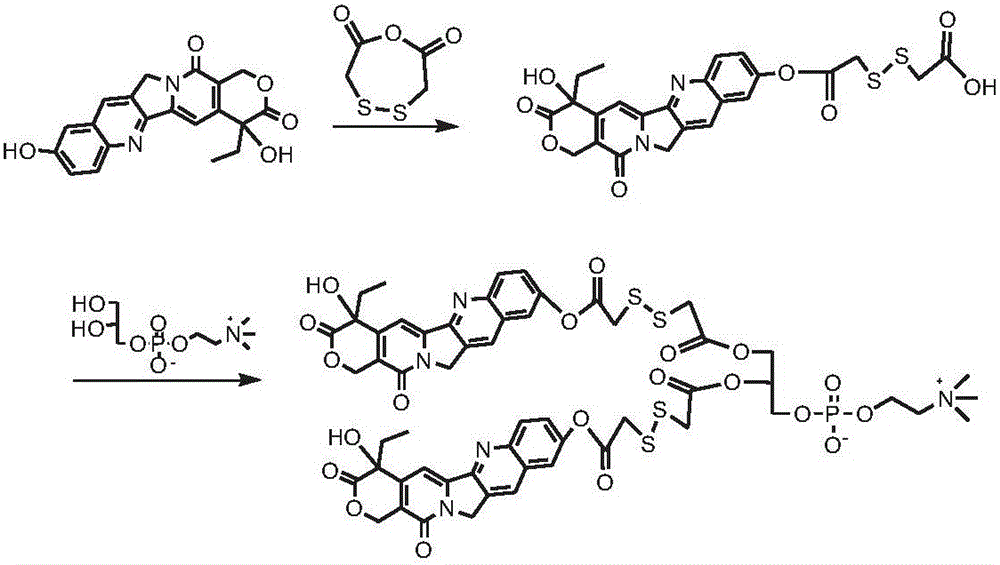
[0122] Synthesis of two (hydroxycamptothecin-10-dithiodiacetic acid) phosphatidylcholine compounds (see the synthetic route figure 1 )
[0123] Dissolve 1g of 10-hydroxycamptothecin and 2g of dithiodiacetic anhydride in 30mL of pyridine, react at 40°C for 48h; remove the solvent by rotary evaporation, precipitate in cold ether, wash with dilute hydrochloric acid, and obtain the intermediate product hydroxycamptothecin-10 - Dithiodiacetate monoester 0.81 g.
[0124] Dissolve 0.8g of the intermediate product hydroxycamptothecin-10-dithiodiacetic acid monoester in 20mLDMSO, add 0.6g of CDI, activate for 1h, add 0.4g of GPC and 0.6g of DBU, react at room temperature for 24h, and the resulting reaction solution is passed through column chromatography After purification, 0.56 g of the product bis(hydroxycamptothecin-10-dithiodiacetic acid) phosphatidylcholine compound was obtained.
[0125] 1 HNMR (500MHz, CD 3 OD: CDCl 3 1:1): δ8.07-7.43 (8H, m), 6.74 (2H, s), 6.51 (2H, s), 4....
Embodiment 2
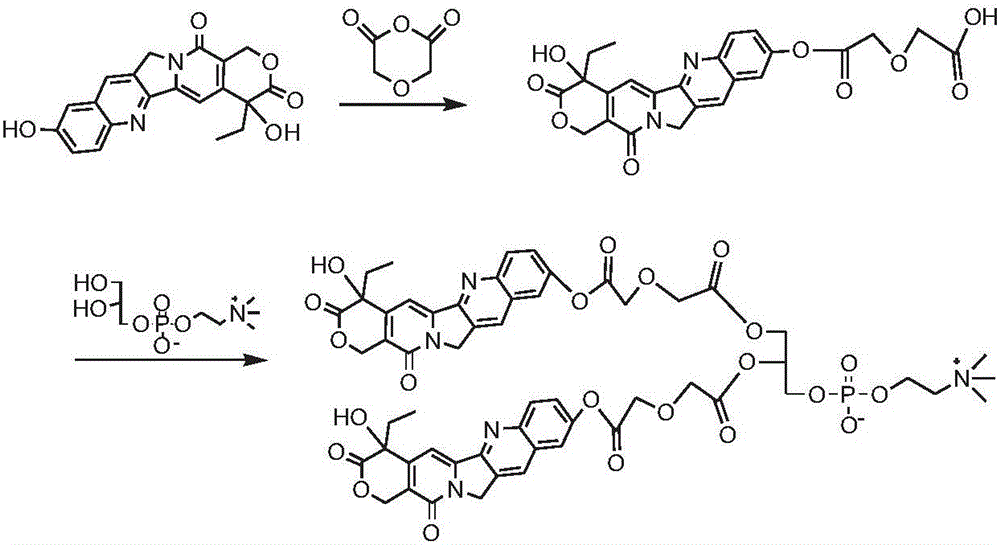
[0127] Synthesis of bis(hydroxycamptothecin-10-diglycolic acid) phosphatidylcholine compound (see the synthetic route figure 2 )
[0128] Dissolve 1g of 10-hydroxycamptothecin and 3g of diglycolic anhydride in 30mL of pyridine, react at 40°C for 48h; remove the solvent by rotary evaporation, precipitate in cold ether, wash with dilute hydrochloric acid, and obtain the intermediate product hydroxycamptothecin-10-di Glycolic acid monoester 0.85g. Take 0.8g of the intermediate product hydroxycamptothecin-10-diglycolic acid monoester, dissolve it in 20mL of DMSO, add 0.8g of CDI, activate for 1h, add 0.4g of GPC and 0.8g of DBU, react at room temperature for 24h, and the resulting reaction solution passes through the column layer Analysis and purification yielded 0.51 g of the product bis(hydroxycamptothecin-10-diglycolic acid) phosphatidylcholine compound.
[0129] 1 HNMR (500MHz, CD 3 OD: CDCl 3 1:1): δ8.07-7.43 (8H, m), 6.74 (2H, s), 6.51 (2H, s), 4.75 (4H, s), 4.64-3.77 ...
Embodiment 3
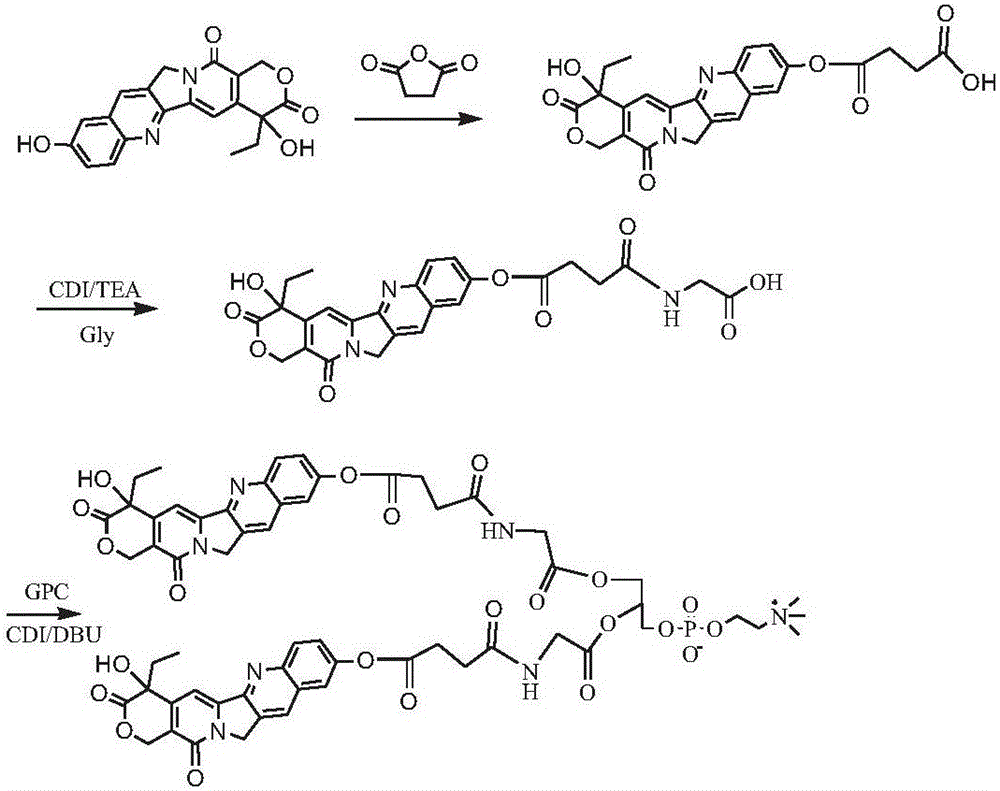
[0131] Synthesis of bis(hydroxycamptothecin-10-succinyl-glycyl)phosphatidylcholine compound (synthetic route see image 3 )
[0132] Dissolve 1g of 10-hydroxycamptothecin and 1.5g of succinic anhydride in 30mL of pyridine, react at 40°C for 48h; remove the solvent by rotary evaporation, precipitate in cold ether, wash with dilute hydrochloric acid, and obtain the intermediate product hydroxycamptothecin-10-succinic acid Monoester 0.75g. Dissolve 0.7 g of the intermediate product hydroxycamptothecin-10-succinic acid monoester in 20 mL of DMF, add 0.5 g of CDI and 0.5 g of TEA, activate for 1 h, then add 1.0 g of glycine, react at room temperature for 24 h, and separate the reaction solution by column chromatography to obtain hydroxycamptothecin Dentin-10-succinyl-glycine 0.53 g. The above product was dissolved in 10 mL of dimethyl sulfoxide, added CDI0.6g, activated for 1h, added GPC0.25g and DBU0.6g, reacted at room temperature for 24h, precipitated in cold ether, separated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com