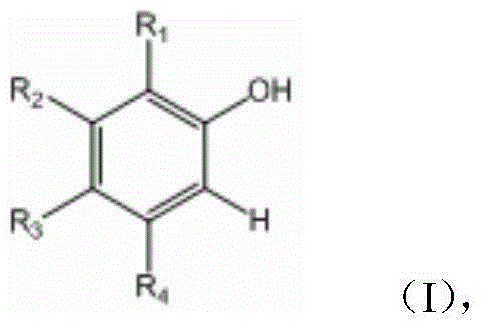
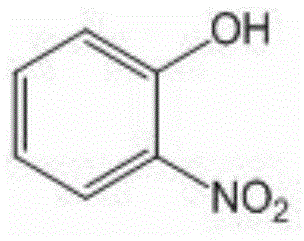
O-nitrophenol and its derivative synthesis method
A technology of o-nitrophenol and a synthesis method, which is applied in the field of organic compound synthesis, can solve the problems of strong acid environment pollution, long synthesis steps, and complexity, and achieves the effects of good selectivity and good substrate adaptability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Take phenol as raw material.
[0036] (2) Add 1.2mmol of phenol, 2mmol of tripotassium phosphate, 0.1mmol of 2-pyridinecarboxylic acid, 1mmol of 2-bromopyridine, 0.05mmol of cuprous iodide, and 2ml of DMSO into a 25ml three-necked flask. Under the protection of argon, the mixture was refluxed at 90°C for 24 hours, and then the reaction was detected by TLC. The resulting mixed solution was extracted with ethyl acetate, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a light yellow solid 2-(phenoxy yl) pyridine (95% yield).
[0037] (3) Add 0.5 mmol of 2-(phenoxy)pyridine, 0.05 mmol of palladium diacetate, 1.0 mmol of tert-butyl nitrite and 5 ml of 1,2-dichloroethane into a 25 ml sealed pressure vessel in sequence. The mixture was heated and reacted in an 80°C oil bath for 24 hours. After the end of the TLC detection reaction, the reaction solution was diluted with dichloromethane and filtered to obtain a clear liqui...
Embodiment 2
[0042] (1) Take phenol as raw material.
[0043] (2) Add 1.2mmol of phenol, 1.8mmol of tripotassium phosphate, 0.1mmol of 2-pyridinecarboxylic acid, 0.6mmol of 2-bromopyridine, 0.05mmol of cuprous iodide, and 4ml of DMSO into a 50ml three-necked flask. The mixture was refluxed at 90°C for 24 hours under the protection of nitrogen, and TLC detected that the reaction was complete. The resulting mixed solution was extracted with ethyl acetate, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a light yellow solid 2-(phenoxy) Pyridine (95% yield).
[0044](3) Add 0.5mmol of 2-(phenoxy)pyridine, 0.05mmol of palladium diacetate, 0.5mmol of tert-butyl nitrite and 5ml of 1,2-dichloroethane into a 25ml sealed pressure vessel in sequence. The mixture was heated and reacted in an 80°C oil bath for 24 hours. After the end of the TLC detection reaction, the reaction solution was diluted with dichloromethane and filtered to obtain a clear li...
Embodiment 3
[0049] (1) Take phenol as raw material.
[0050] (2) Add 1.2mmol of phenol, 3mmol of tripotassium phosphate, 0.3mmol of 2-pyridinecarboxylic acid, 0.6mmol of 2-bromopyridine, 0.012mmol of cuprous iodide, and 5ml of DMSO into a 50ml three-necked flask. The mixture was refluxed at 90°C for 30 hours under the protection of argon, and TLC detected that the reaction was complete. The resulting mixed solution was extracted with ethyl acetate, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a light yellow solid 2-phenoxypyridine (95% yield).
[0051] (3) Add 0.5 mmol of 2-phenoxypyridine, 0.08 mmol of palladium trifluoroacetate, 1.5 mmol of tert-butyl nitrite and 5 ml of 1,2-dichloroethane into a 25 ml sealed pressure vessel in sequence. The mixture was heated and reacted in an 80°C oil bath for 24 hours. After the end of the TLC detection reaction, the reaction solution was diluted with dichloromethane and filtered to obtain a clea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com