Composite microspheres for ordered release of growth factors and antibiotics, preparation method and application
A growth factor and composite microsphere technology, applied in the field of biomedical materials, can solve the problems of a large amount of burst release, the generation of acidic environment by microsphere degradation products, and the limitation of PLGA microspheres, and achieve good antibacterial, anti-inflammatory, and good biodegradability. , the effect of reducing the bursting phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: A preparation method for ordered release of growth factor and antibiotic composite microspheres
[0023] 1) Dissolve 80 mg of PLGA polymer in 2 mL of dichloromethane to obtain an oil phase; dissolve 2 μg of bFGF in 200 μL of deionized water and slowly add it to the oil phase, and emulsify at 20,000 rpm / min for 1 min with a high-speed homogenizer Get colostrum W1 / O;
[0024] 2) Dissolve 250 mg PVA in 25 mL deionized water to obtain a 1% PVA aqueous solution; dissolve 50 mg GC and 125 mg chlorhexidine acetate (CHA) in the above PVA aqueous solution to obtain a GC / CHA / PVA solution;
[0025] 3) Slowly add the colostrum W1 / O obtained in step 1) to the GC / CHA / PVA solution obtained in step 2), and use a high-speed homogenizer to stir at 12000 rpm / min for 3 minutes to obtain double milk W1 / O / W2;
[0026] 4) The double emulsion was magnetically stirred at 800 r / min for 4 hours, centrifuged and washed with deionized water for 5 times, and freeze-dried to obtain comp...
Embodiment 2
[0027] Example 2: A preparation method for ordered release of growth factor and antibiotic composite microspheres
[0028] 1) Dissolve 100 mg of PLGA polymer in 2 mL of dichloromethane to obtain an oil phase; dissolve 2 μg of bFGF in 200 μL of deionized water and slowly add it to the oil phase, and emulsify at 15,000 rpm / min with a high-speed homogenizer1 min to get colostrum W1 / O;
[0029] 2) Add 500 mg PVA to 25 mL deionized water to obtain a 2% PVA aqueous solution; dissolve 125 mg GC and 25 mg CHA in the above PVA aqueous solution to obtain a GC / CHA / PVA solution;
[0030] 3) Slowly add the colostrum W1 / O obtained in step 1) to the GC / CHA / PVA solution obtained in step 2), and use a high-speed homogenizer to stir at 8000 rpm / min for 3 minutes to obtain double milk W1 / O / W2 ;
[0031] 4) The double emulsion was magnetically stirred at 800 r / min for 4 hours, centrifuged and washed with deionized water for 5 times, and freeze-dried to obtain composite microspheres co-loaded wi...
Embodiment 3
[0032] Example 3: In vitro release experiment of ordered release growth factor and antibiotic composite microspheres
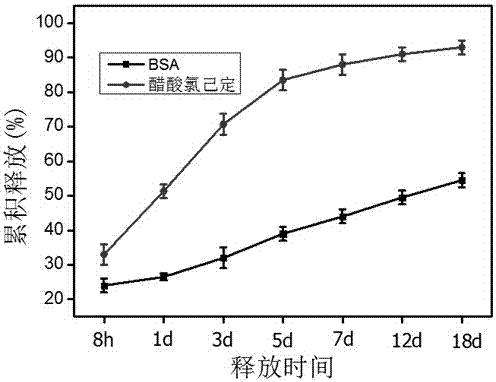
[0033] 1) Weigh 10 mg of composite microspheres and put them in 1 mL of PBS solution (pH 7.4), shake at 37°C and 110 rpm, use BCA kit to measure the release of growth factors and calculate their cumulative release ; Using a UV-Vis spectrophotometer at 253 nm to measure the release of chlorhexidine acetate and calculate its cumulative release rate. Three parallel samples were performed, and the results were expressed as mean ± standard deviation.
[0034] 2) Experimental results: by figure 2 It can be seen that the release rate of chlorhexidine acetate is fast, with a cumulative release of more than 90% within one week, and the release of bFGF is slow, indicating that the composite microspheres can release growth factors and antibiotics in an orderly manner, so as to quickly inhibit inflammation and continuously induce disease-deficient tissue. purpose of re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com